Methods of treating cancer with aryl hydrocarbon receptor antagonists
A technology of aryl hydrocarbon receptors and antagonists, which can be used in antineoplastic drugs, pharmaceutical formulations, and medical raw materials derived from mammals, and can solve problems such as detection and treatment difficulties
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
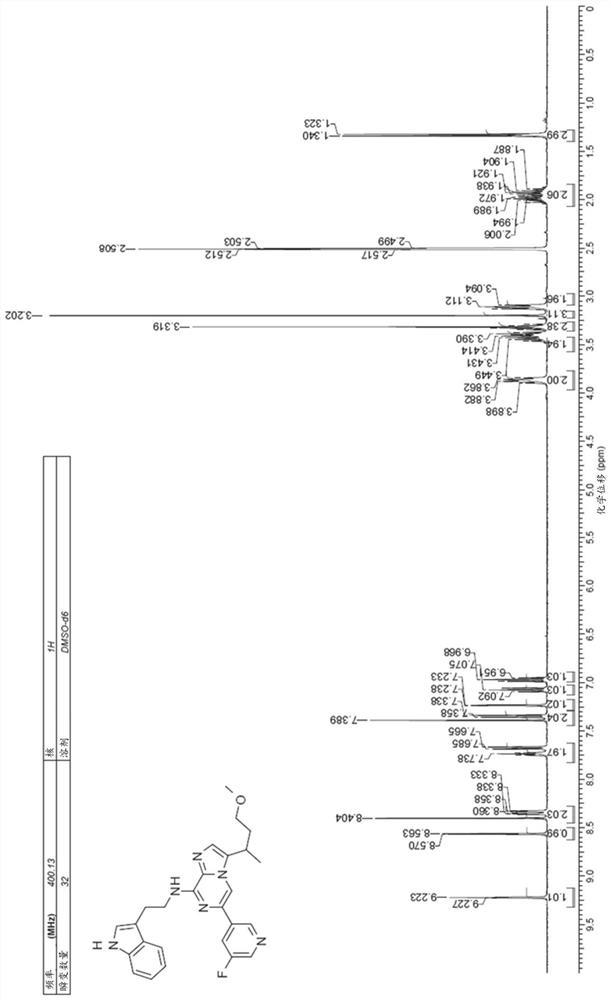
[0718] The synthesis of embodiment 1. compound (5)
[0719] Compound (5) can be synthesized, for example, using the Hartwig-Buchwald amination method involving the coupling of a haloimidazopyridine precursor to a protected 2-aminoethylindole, as shown in Scheme 8 below.
[0720] Option 8
[0721]
[0722] For example, compound (5) can be produced by deprotecting the resulting adduct using conventional deprotection methods known in the art.
[0723] Alternatively, the tandem amination-hydrolysis procedure outlined in Scheme 9 below can be used to synthesize compounds (5).
[0724] Option 9
[0725]
Embodiment 2
[0726] Embodiment 2. the synthesis of compound (16)
[0727] In a manner similar to that described in Example 1, compound (16) can be synthesized by the Hartwig-Buchwald method followed by Suzuki coupling and indole deprotection, as shown in Scheme 10 below.
[0728] Scheme 10
[0729]
Embodiment 3
[0730] Example 3. Compounds (5) and (16) Ability to Expand Hematopoietic Stem Cells
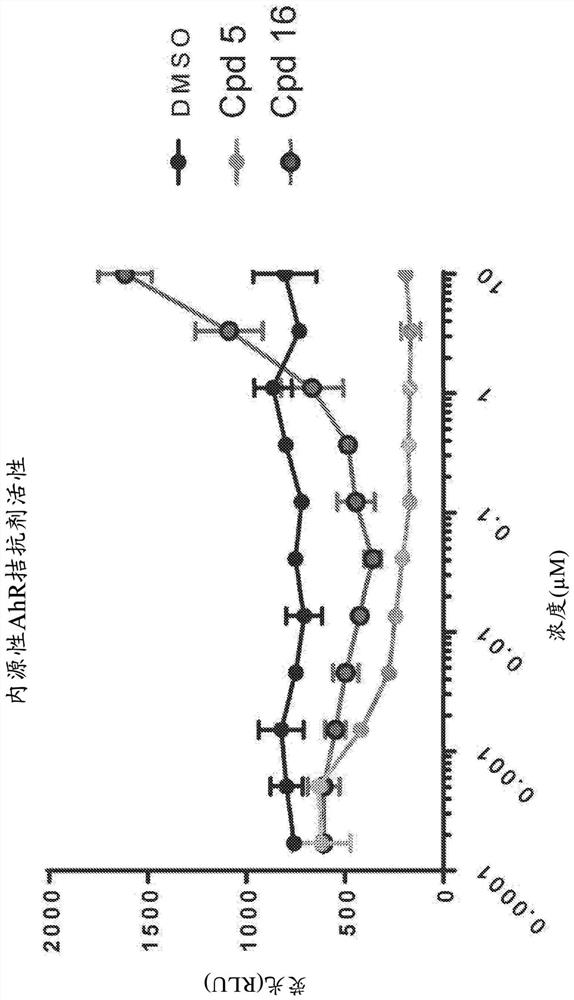
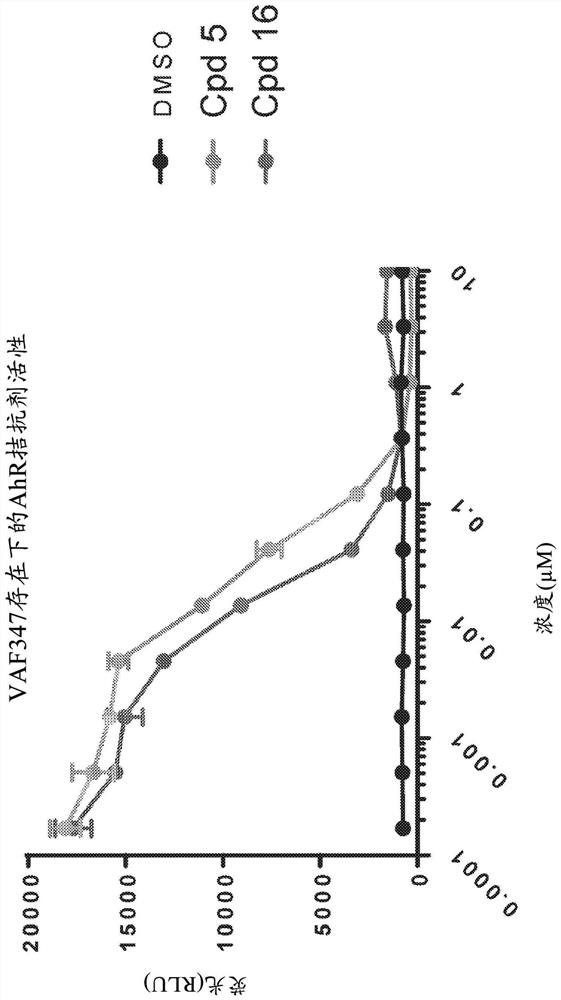
[0731] In order to determine the ability of compounds (5) and (16) to inhibit aryl hydrocarbon receptor activity and induce hematopoietic stem cell proliferation, a series of HSC expansion experiments were performed. In a first experiment, compounds (5) and (16) were assessed for their ability to attenuate aryl hydrocarbon receptor signaling. To this end, HepG2 hepatocytes were transiently transfected with a luciferase reporter gene construct under the control of a promoter responsive to aryl hydrocarbon receptor signaling. Cells were seeded in microtiter plates at a density of 25,000 cells per well. In the absence of the aryl hydrocarbon receptor agonist VAF347 (80nM) ( figure 1 ) or exists ( figure 2 ), HepG2 cells were immediately treated with compound (5) or (16). Luciferase activity was subsequently assayed six hours after plating.
[0732] Such as figure 1 and 2 It was shown tha...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com