Method for preparing clioquinol and diiodohydroxyquinoline by one-pot method
A technology for clioquinol and diiodoquinoline is applied in the field of preparing clioquinol and diiodoquine in one pot, and can solve the problems of poor atom economy, harsh reaction conditions, increasing the difficulty of purification, and the like. High productivity and environmentally friendly results
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
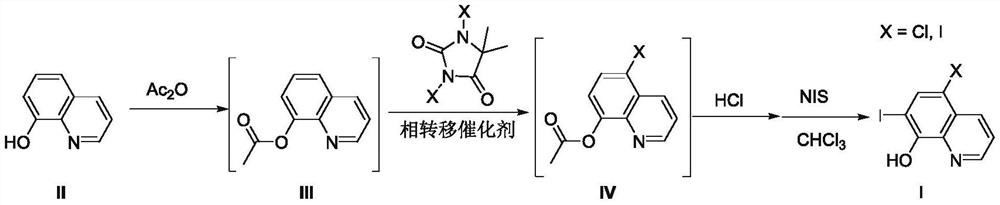
[0025] Adopt the inventive method to prepare 5-chloro-7-iodoquinolin-8-alcohol (Ia), i.e. clioquinol, the reaction formula is as follows:
[0026]
[0027] S1: Put 8-hydroxyquinoline (compound II) (7.25g, 50mmol), acetic anhydride (60mL) and glacial acetic acid (20mL) into a 150mL reaction flask, reflux for 5h, and the reaction solution was concentrated to dryness under reduced pressure to obtain White powdery solid quinolin-8-yl acetate (compound III), directly carried out the next step reaction without purification;
[0028] S2: Add the quinolin-8-yl acetate (compound III), dichlorohydantoin (6.90 g, 35 mmol), benzyltriethylammonium chloride (TEBA) ( 2.28g, 10mmol) and water 75mL, stirred and reacted at 50°C for 12h, filtered the reaction liquid, washed with a small amount of ice water to obtain off-white powdery solid 5-chloroquinolin-8-yl acetate (Compound IVa), the wet product was not purified proceed directly to the next reaction;
[0029] S3: 5-chloroquinolin-8-yl ...
Embodiment 2
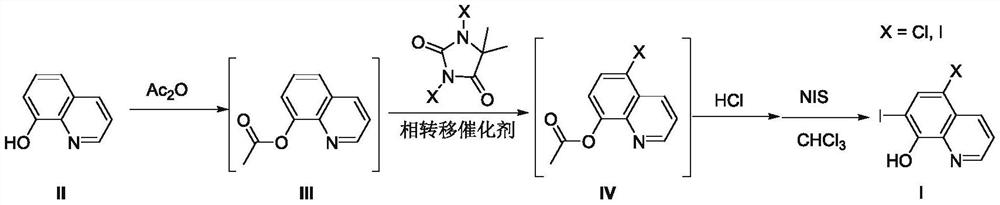
[0035] Adopt the inventive method to prepare 5,7-diiodoquinolin-8-alcohol (Ib), i.e. diiodoquinoline, reaction formula is as follows:
[0036]
[0037] S1: Put 8-hydroxyquinoline (compound II) (7.25g, 50mmol), acetic anhydride (60mL) and glacial acetic acid (20mL) into a 150mL reaction flask, reflux for 5h, and the reaction solution was concentrated to dryness under reduced pressure to obtain White powdery solid quinolin-8-yl acetate (compound III), directly carried out the next step reaction without purification;
[0038] S2: Put quinolin-8-yl acetate (compound III), diiodohydantoin (13.30g, 35mmol), TEBA (2.28g, 10mmol) and water 75mL prepared in step S2 into a 150mL reaction flask, 50 Stir the reaction at ℃ for 12 hours, filter the reaction solution, wash with a small amount of ice water to obtain off-white powdery solid 5-chloroquinolin-8-yl acetate (compound IVb), and the wet product is directly carried out to the next step reaction without purification.
[0039] S3: ...
Embodiment 3
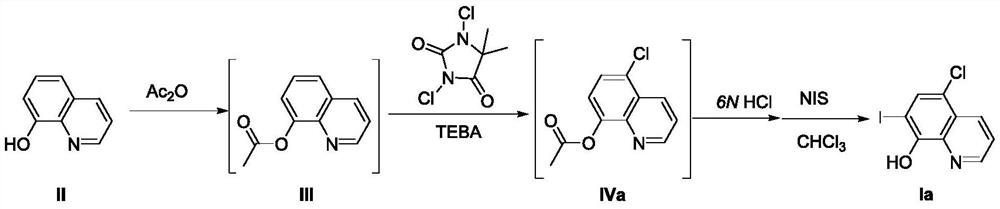
[0045] Adopt the inventive method to prepare 5-chloro-7-iodoquinolin-8-alcohol (Ia), i.e. clioquinol, comprises the steps:
[0046] S1: Put 8-hydroxyquinoline (compound II) (7.25g, 50mmol), acetic anhydride (60mL) and glacial acetic acid (20mL) into a 150mL reaction flask, reflux for 5h, and the reaction solution was concentrated to dryness under reduced pressure to obtain White powdery solid quinolin-8-yl acetate (compound III), directly carried out the next step reaction without purification;
[0047] S2: Put quinolin-8-yl acetate (compound III), dichlorohydantoin (6.90g, 35mmol), TEBA (2.28g, 10mmol) and water 75mL prepared in step S1 into a 150mL reaction bottle, 100 Stir the reaction at ℃ for 12 h, filter the reaction solution, and wash with a small amount of ice water to obtain 5-chloroquinolin-8-yl acetate (compound IVa) as a yellow powder solid, and the wet product is directly carried out to the next step reaction without purification.
[0048] S3: 5-chloroquinolin-8-...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com