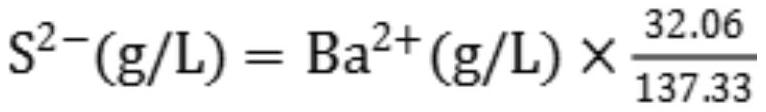Method for rapidly analyzing and detecting impurity sodium sulfide in barium sulfide solution
A rapid analysis and detection method technology, which is applied in the direction of chemical analysis by titration, analysis by making materials undergo chemical reactions, and material analysis by observing the impact on chemical indicators, etc., which can solve volatility and low production efficiency , limit the application of mechanization, automation and informatization in the compounding process, and achieve the effects of sharp color change, easy observation, and simple operation methods
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0041] The rapid analysis detection of impurity sodium sulfide in the barium sulfide solution of embodiment 1
[0042] Use a 10ml pipette to accurately pipette 10ml of barium sulfide aqueous solution to a 500ml volumetric flask and shake well; add V to a 250ml Erlenmeyer flask 2 =5ml iodine standard titration solution (concentration C 2 =0.1010mol / L), 10ml water and 10ml hydrochloric acid solution, adjust the pH of the solution to be neutral or slightly acidic; then accurately pipette 10ml sample solution from the 500ml volumetric flask with a pipette and add it to the 250ml Erlenmeyer flask, while Shake constantly; use a concentration of C 1 =0.05025mol / L sodium thiosulfate standard solution titration, when the solution is titrated to light yellow, add 2ml starch indicator, continue to titrate with sodium thiosulfate standard solution until the blue color disappears, and consume the volume V of sodium thiosulfate 1 = 6.46ml; then pipette 10ml of the sample solution from the...
Embodiment 2
[0051] The rapid analysis detection of impurity sodium sulfide in the barium sulfide solution of embodiment 2
[0052] Use a 10ml pipette to accurately pipette 10ml of barium sulfide aqueous solution to a 500ml volumetric flask, shake well; put V in a 250ml conical flask 2 =5ml iodine standard titration solution (concentration C 2 =0.1010mol / L), 20ml water and 10ml hydrochloric acid solution, adjust the pH of the solution to be neutral or slightly acidic; then accurately pipette 10ml sample solution from the 500ml volumetric flask with a pipette and add it to the 250ml Erlenmeyer flask, while Shake constantly; use a concentration of C 1 =0.05025mol / L sodium thiosulfate standard solution titration, when the solution is titrated to light yellow, add 2ml starch indicator, continue to titrate with sodium thiosulfate standard solution until the blue color disappears, and consume the volume V of sodium thiosulfate 1 = 5.84ml; then pipette 10ml of the sample solution from the 500ml...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com