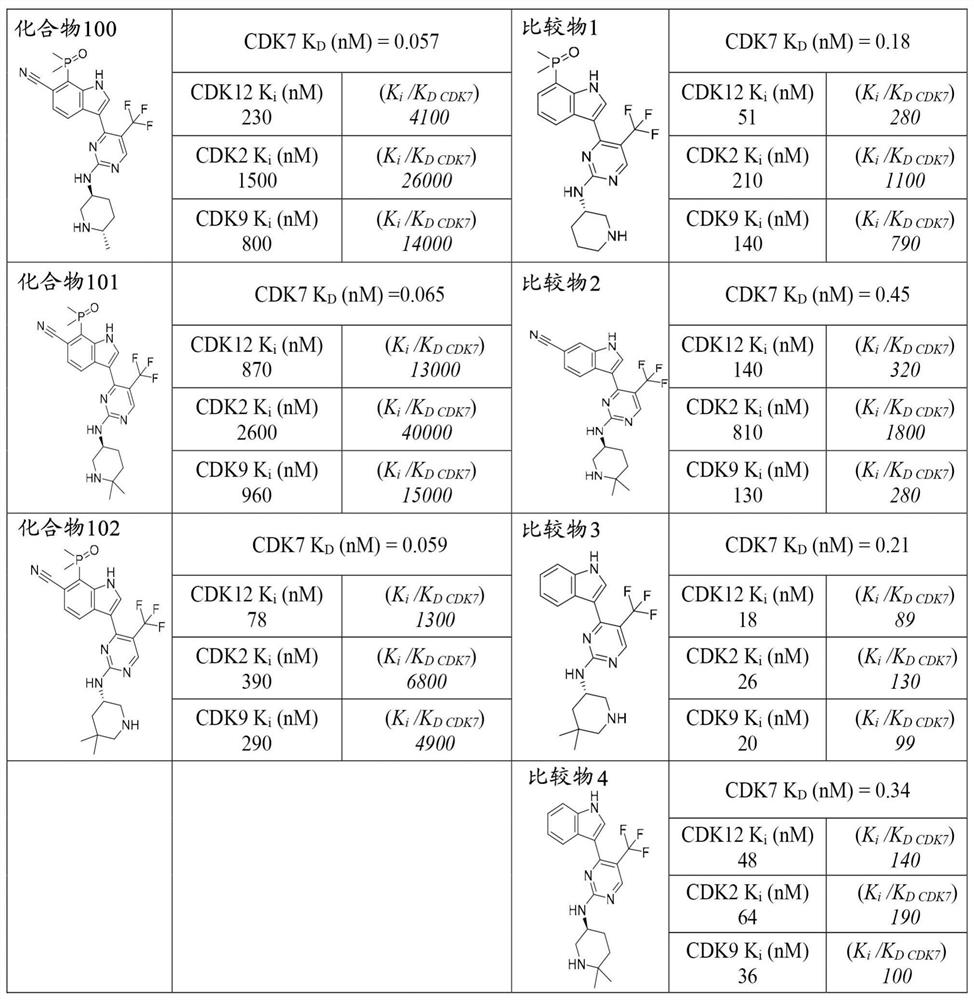
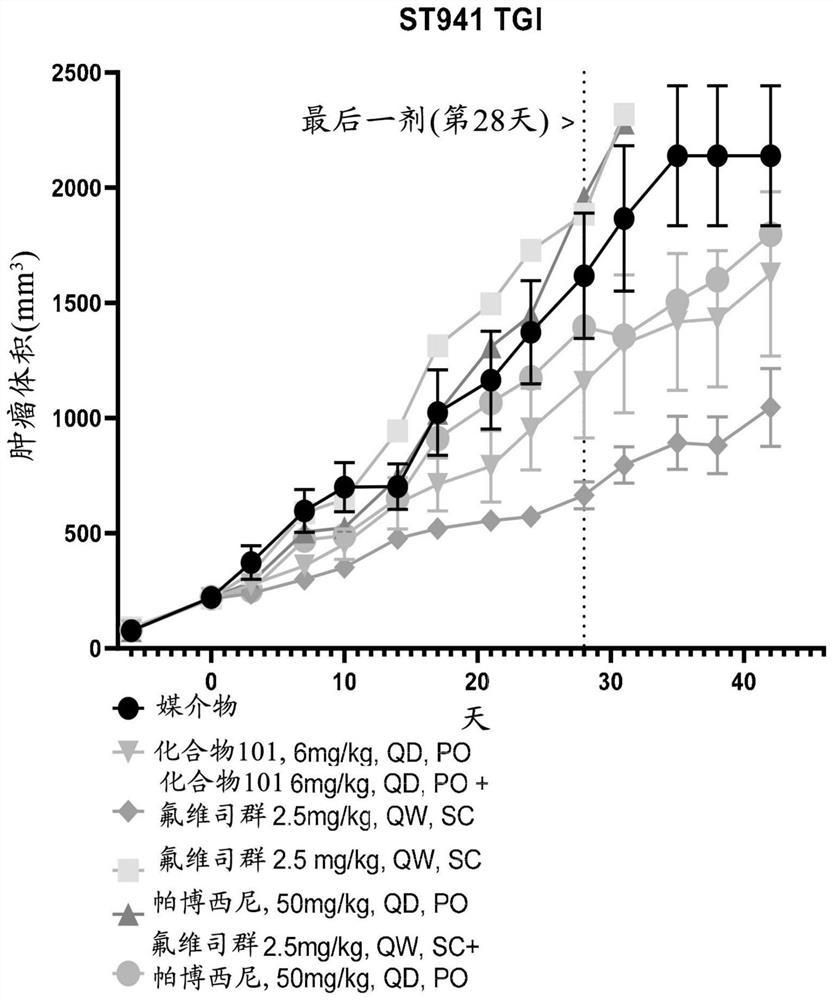
Methods of treating cancer in biomarker-identified patients with non-covalent inhibitors of cyclin-dependent kinase 7 (CDK7)
A technology of solvates and uses, applied in the fields of compounds of elements of group 5/15 of the periodic table, organic chemical methods, chemical instruments and methods, etc., can solve problems such as few commercial applications
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0099] In case of doubt, a compound of formula (I), (Ia) or any specified form of its species may be contained in a pharmaceutical composition for use and administered according to the invention (for example, in an effective amount (for example, a therapeutically effective quantity)). The pharmaceutical compositions used in the methods of the present invention can be prepared by relevant methods known in the field of pharmacology. Generally, such preparation method comprises the following steps: the compound described herein, including the compound of formula (I), (Ia), its species or its specified form (for example, its pharmaceutically acceptable salt, solvate, stereo isomers, tautomers, or isotopic forms thereof) are combined with a carrier and / or one or more other active ingredients (for example, a second agent as described herein) and / or auxiliary ingredients, and then, If necessary and / or desired, molding and / or packaging of the product are carried out in desired single...
Embodiment 1
[0112] Example 1: Benzyl (2R,5R)-5-amino-2-methyl-piperidine-1-carboxylate and (2S,5S)-5-amino-2-methyl-piperidine-1-carboxylate Synthesis of esters
[0113] Step 1: Benzyl 5-(tert-butoxycarbonylamino)-2-methyl-piperidine-1-carboxylate
[0114]
[0115] At 0°C, we prepared a solution containing commercially available racemic trans-N-(6-methyl-3-piperidinyl)carbamate tert-butyl ester (5 g, 23.33 mmol, 1 eq.) and NaHCO 3 (13.72g, 163.32mmol, 7eq) in tetrahydrofuran (THF; 50mL) and H 2 To a solution in O (50 mL) was added CbzCl (5.97 g, 35.00 mmol, 4.98 mL, 1.5 equiv) dropwise. The mixture was stirred at 15 °C for 2 hours, then poured into water (50 mL) and extracted with ethyl acetate (EtOAc; 50 mL x 3). The combined organic layers were washed with brine (50mL×3), washed with Na 2 SO 4 Dry and filter. The filtrate was concentrated under reduced pressure, and the residue was subjected to medium pressure liquid chromatography (MPLC; SiO 2 , PE:EtOAc=5:1 to 1:1) to afford...
Embodiment 2
[0119] Example 2: 7-Dimethylphosphoryl-3-[2-[[(3S,6S)-6-methyl-3-piperidinyl]amino]-5-(trifluoromethyl)pyrimidine-4 Synthesis of -yl]-1H-indole-6-carbonitrile (compound 100)
[0120] Step 1: (2S,5S)-5-[[4-(7-Chloro-6-cyano-1H-indol-3-yl)-5-(trifluoromethyl)pyrimidin-2-yl]amino ]-2-Methyl-piperidine-1-carboxylic acid benzyl ester
[0121]
[0122] We added 7-chloro-3-[2-chloro-5-(trifluoromethyl)pyrimidin-4-yl]-1H-indole-6-carbonitrile (0.81 g, 2.27 mmol, 1 equivalent), (2S ,5S)-5-Amino-2-methyl-piperidine-1-carboxylic acid benzyl ester (732.20 mg, 2.95 mmol, 1.3 equiv) and N,N-diisopropylethylamine (DIEA or DIPEA; 879.41 mg, 6.80 mmol, 1.19 mL, 3 eq) in N-methyl-2-pyrrolidone (NMP; 8 mL) was stirred at 140 °C for 1 h. The reaction mixture was treated with H 2 Diluted with O (100 mL) and extracted with EtOAc (50 mL x 2). The combined organic layers were washed with brine (100mL x 2), washed with Na 2 SO 4 Dry, filter, and concentrate under reduced pressure to give a r...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com