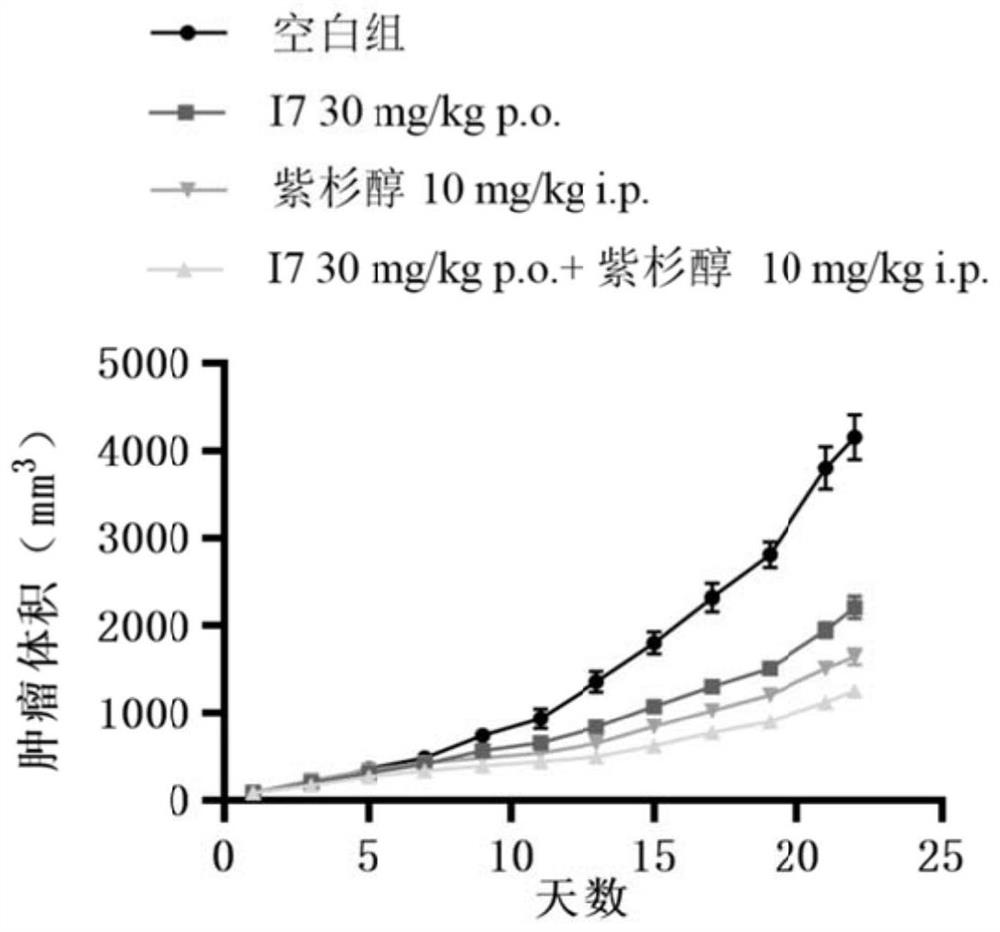
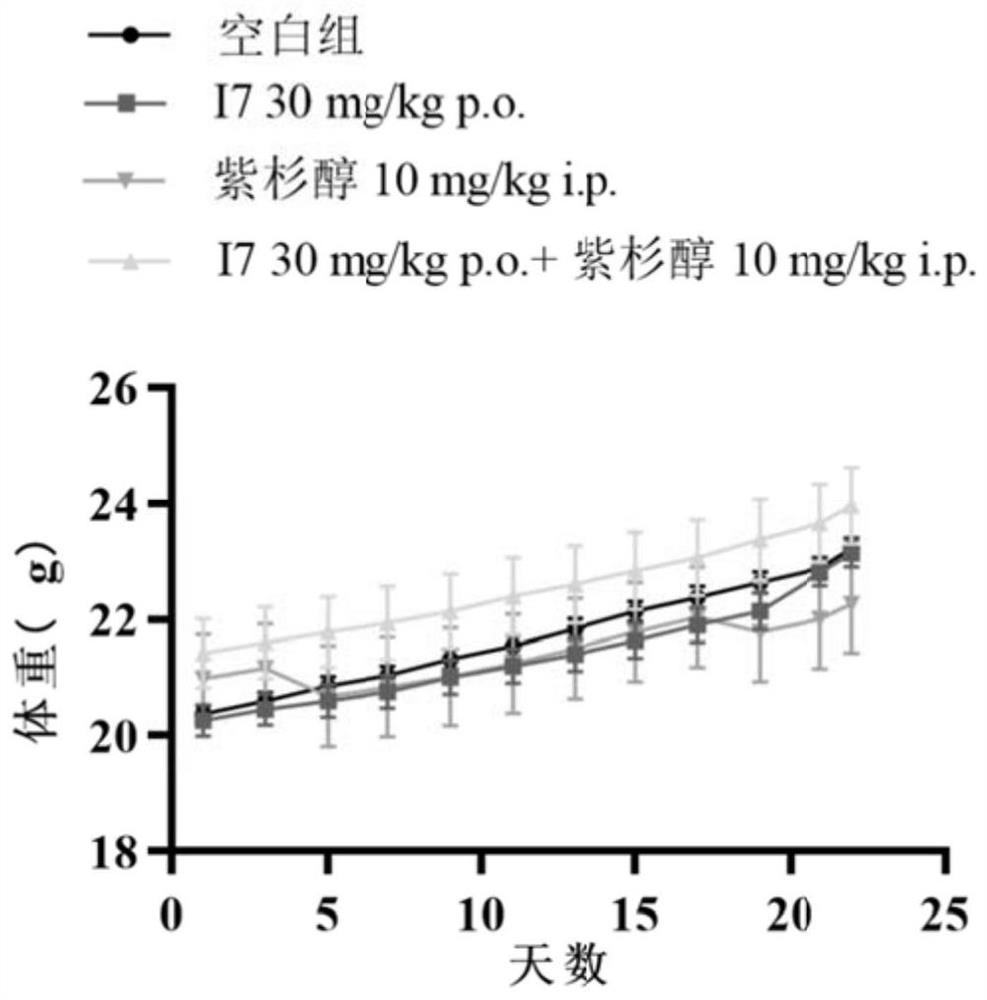
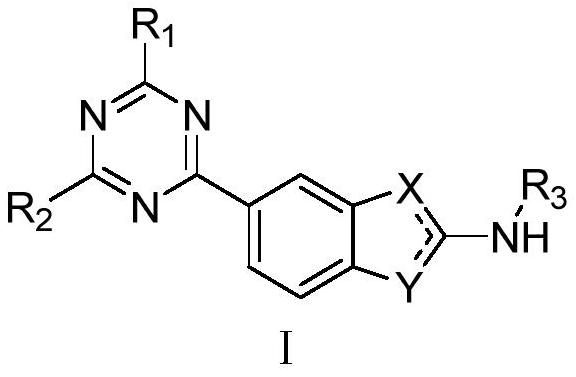
PI3Kalpha selective inhibitor and preparation method and application thereof
A technology selected from C3-C8, applied in non-central analgesics, anti-inflammatory agents, pharmaceutical formulations, etc., can solve the problems of unsatisfactory clinical results and achieve good inhibitory effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0116] 1) 5-(4-(4-(methylsulfonyl)piperazin-1-yl)-6-morpholino-1,3,5-triazin-2-yl)benzo[d]oxazole -Synthesis of 2-amine (I-1):
[0117]
[0118] Dissolve intermediates M2 (100mg, 1.0eq.) and M12 (91mg, 1.0eq.) in ethylene glycol dimethyl ether, dissolve sodium carbonate (74.2mg, 2.0eq.) as a base with a small amount of water and add to the reaction flask , added tetrakis(triphenylphosphine)palladium (40.5mg, 0.1eq.) as a catalyst, protected by argon, and reacted at 92°C for 24h. The reaction was monitored by TLC. After the reaction was completed, it was filtered while hot to remove salt and metal catalysts. The remaining reaction solution was extracted with DCM for 3 times. The organic layer was dried with anhydrous sodium sulfate, concentrated under reduced pressure, and further purified by flash preparative liquid chromatography, white Solid 109mg, yield 68%, 1 H NMR (400MHz, DMSO-d 6 )δ8.16(d, J=1.7Hz, 1H), 8.08(dd, J=8.3, 1.7Hz, 1H), 7.53(s, 2H), 7.39(d, J=8.3Hz, 1H)...
Embodiment 2
[0120] 2) 5-(4-(4-ethylpiperazin-1-yl)-6-morpholino-1,3,5-triazin-2-yl)benzo[d]oxazol-2-amine Synthesis of (I-2):
[0121]
[0122] With reference to the synthesis of Example 1, white solid, yield 82%, 1 H NMR (400MHz, DMSO-d 6 )δ8.13(d, J=1.6Hz, 1H), 8.06(dd, J=8.4, 1.7Hz, 1H), 7.52(s, 2H), 7.37(d, J=8.4Hz, 1H), 3.79– 3.64(m,12H),2.41–2.35(m,6H),1.03(t,J=7.1Hz,3H)ppm; HRMS(ESI)m / z calcd.forC 20 h 26 N 8 o 2 (M+H)+411.2251, found: 411.2247.
Embodiment 3
[0124] 3) 5-(4-(4-isopropylpiperazin-1-yl)-6-morpholino-1,3,5-triazin-2-yl)benzo[d]oxazole-2- Synthesis of amine (I-3):
[0125]
[0126] With reference to the synthesis of Example 1, white solid, yield 67%, 1 H NMR (400MHz, DMSO-d 6 )δ8.14(d, J=1.6Hz, 1H), 8.06(dd, J=8.4, 1.7Hz, 1H), 7.52(s, 2H), 7.38(d, J=8.4Hz, 1H), 3.86– 3.65(m,12H),2.73-2.66(m,1H),2.51-2.47(m,4H),0.99(d,J=6.5Hz,6H)ppm; HRMS(ESI)m / zcalcd.for C 21 h 28 N 8 o 2 (M+H) + 425.2408,found: 425.2406.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com