Novel C-glycoside glycosyl transferase and application thereof
A technology of glycosyltransferase and carbon glycosides, applied in the field of synthetic biology
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
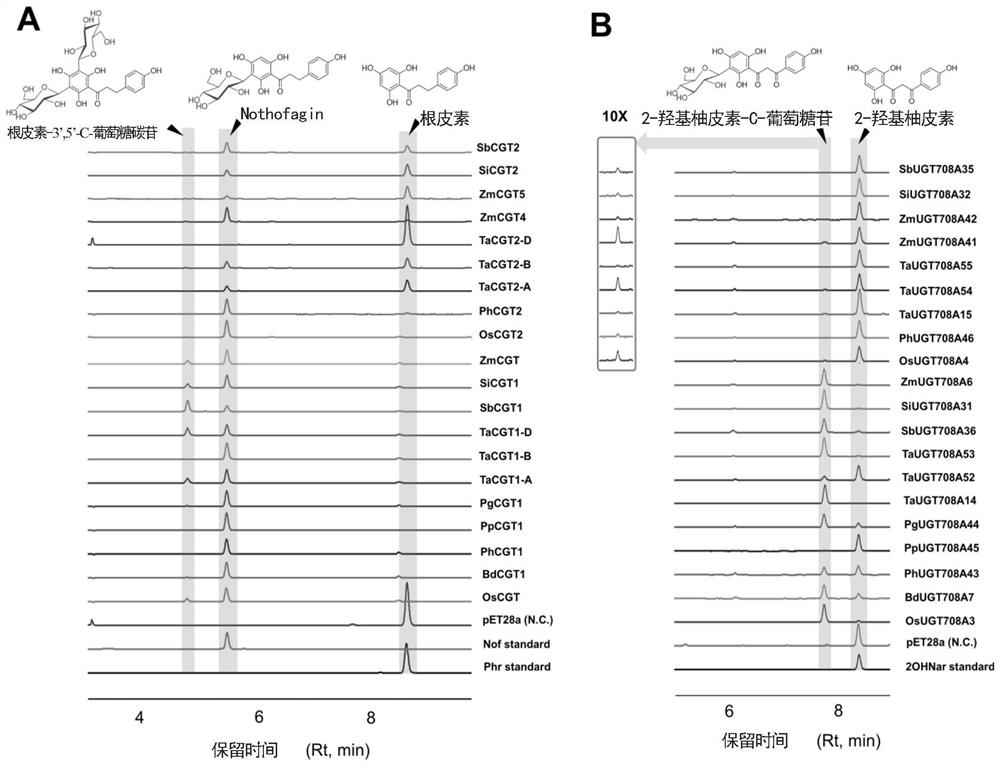
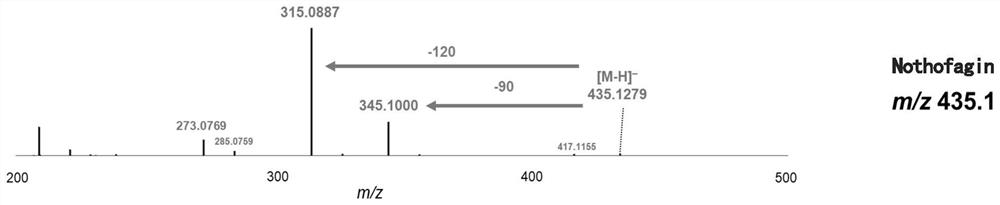
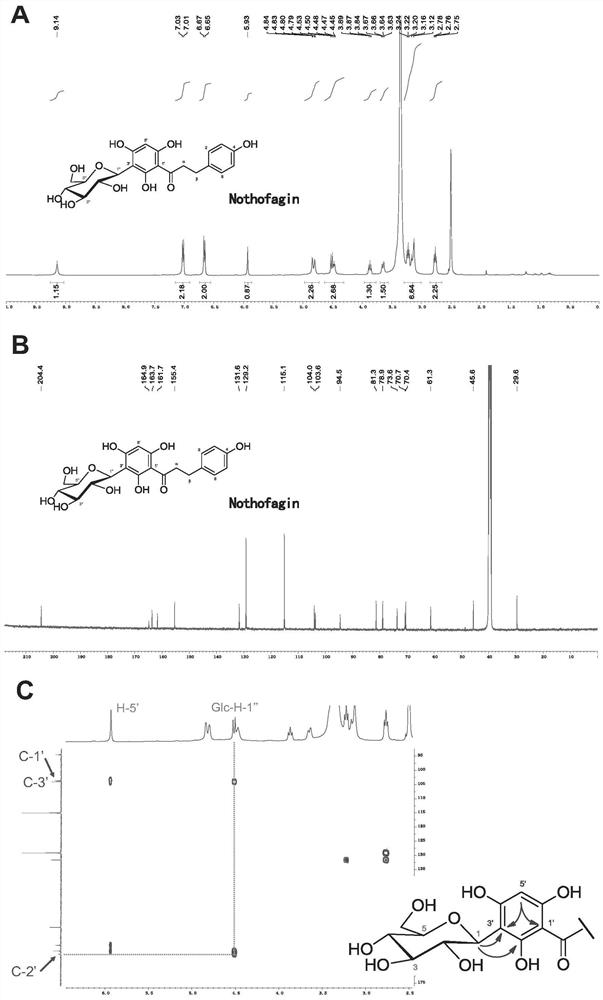
[0104] Embodiment 1, novel uridine diphosphate (UDP)-glycosyltransferase
[0105] 1. Cloning of carboside glycosyltransferase gene and construction of expression plasmid
[0106] Take mature leaves or seedlings of gramineous plants (japonica rice, corn, wheat, sorghum, millet, Brachypodium distachybes, moso bamboo, light bamboo, sargassum), clean the surface stains with clean water, and gently wipe the water on the surface of dry leaves with absorbent paper. stains, weighed for later use. Genomic DNA was extracted according to the instructions provided by the TIANGEN Plant Genome Extraction Kit.
[0107] The amino acid sequence of the described carboside glycosyltransferase is as follows:
[0108] >PhCGT1 (SEQ ID NO: 1)
[0109] MPTSGGRTGDRPHVILLPSAGMGHLVPFSRLAVALSSGHGCDVSVVAVLPTVSAAESRHLQGLLDASPAVRRLDLHLAPFDESEFPGADPFFLRFEAMRRSAPLLGPLLADTGASALVTDIALASVVIPVAKEIHLPCYVLFTASAAMLSFCAYFPTYLDTNAGRVVGDVDIPGVYRIPKASIPQALHDPNHLFTRQFVANGQDLAKADGVLVNTFDALELEAVTALRQGSVAAGFPPVFSVGPLVTVN...
Embodiment 2
[0163] Example 2. Using the glycosyltransferase of the present invention to synthesize vitexin, isovitexin, orientin and isoorientin In this example, PhCGT1 was used to synthesize vitexin and isovitexin in Escherichia coli Isovitexin, orientin and isoorientin
[0164] The predicted biosynthetic pathway of flavone-C-monoglucoside as Figure 11 . Arrows TAL (or PAL), 4CL, CHS, CHI represent the main enzymes responsible for naringenin biosynthesis. Arrows F2H, F3'H are flavanone 2-hydroxylase (F2H) and flavanone 3'-hydroxylase (F3'H), which constitute the backbone of hydroxylated flavanones. C-Glycosyltransferase (CGT) is indicated by the arrow CGT. After the formation of a C-glycosylated intermediate, the dehydration reaction occurs spontaneously in an acidic solvent (arrow H + ) or catalyzed by a dehydratase (arrow DH).
[0165] 1. Plasmid construction
[0166] (1) Construction of the expression cassette for the synthetic precursor gene of the flavanone naringenin
[016...
Embodiment 3
[0203] Embodiment 3, use the glycosyltransferase of the present invention to synthesize Wei Cai Ning-2
[0204] In this example, TaCGT1-D was used to synthesize vicenin-2 in Escherichia coli. The predicted biosynthetic pathway of Wei Cai Ning-2 (pigenin-6,8-diglucoside) is as follows: Figure 12 . TAL (or PAL), 4CL, CHS, CHI represent the main enzymes responsible for naringenin biosynthesis. Arrow F2H is flavanone 2-hydroxylase (F2H), which constitutes the backbone of 2-hydroxyflavanone. C-Glycosyltransferase (CGT) is indicated by the arrow CGT. After the formation of a C-glycosylated intermediate, the dehydration reaction occurs spontaneously in an acidic solvent (arrow H + ).
[0205] 1. Plasmid construction
[0206] (1) The construction of the expression cassette for the synthetic precursor gene of the flavanone naringenin is the same as in Example 2.
[0207] (2) The construction of the flavanone-2-hydroxylase / CPR expression cassette is the same as in Example 2.
...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com