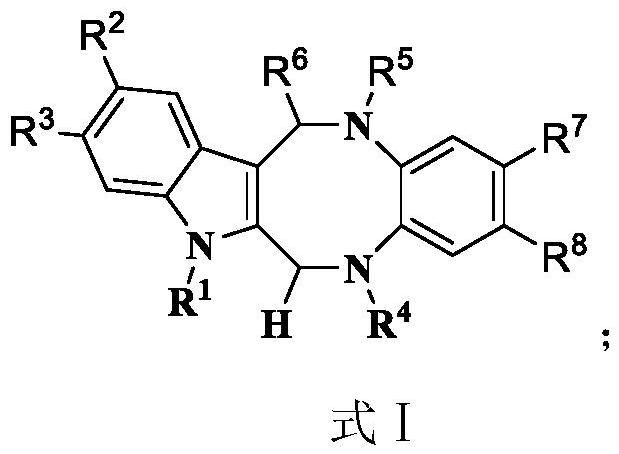
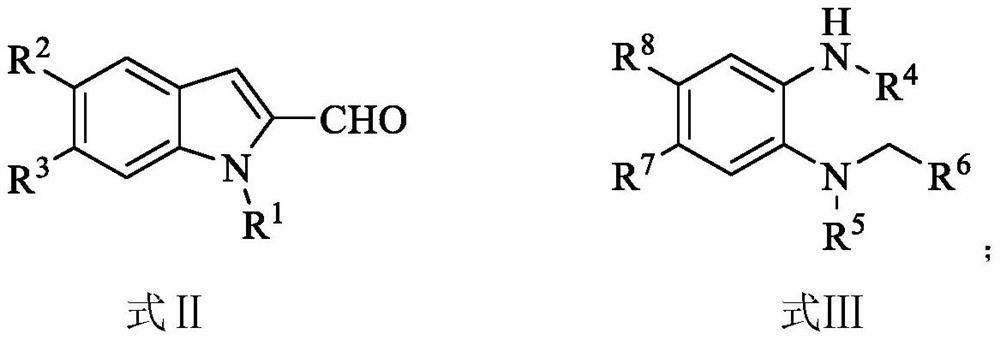
Indole eight-membered middle-ring compound and preparation method thereof
A ring compound and compound technology, applied in the field of indolo eight-membered ring compound and its preparation, can solve the problems of severe reaction conditions, complex synthesis of raw materials, limited substrates, etc., and achieve the effect of mild conditions and simple operation
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0071]
[0072] White solid; 30.2mg, 95% yield; mp 192-194°C; column chromatography yeluent, petroleum ether / EtOAc=250:1; 1 H NMR (500MHz, CDCl 3 )δ7.43(d, J=8.0Hz, 1H), 7.10(d, J=8.2Hz, 1H), 7.05-7.00(m, 1H), 6.96-6.91(m, 1H), 6.85-6.81(m ,2H),6.76-6.68(m,2H),5.45(dd,J=8.2,6.6Hz,1H),5.14(d,J=14.5Hz,1H),4.16(d,J=14.5Hz,1H) ,3.54(s,3H),3.42-3.32(m,1H),3.17-3.08(m,1H),2.92(s,3H),2.46-2.36(m,1H),2.36-2.28(m,1H) ,2.20-2.01(m,2H); 13 CNMR (125MHz, CDCl 3 )δ144.00,143.26,137.08,135.03,126.09,122.96,122.14,120.93,120.80,120.65,118.93(2C),114.01,109.11,60.88,52.71,50.91,44.20,31.28,29.43,24.02;HRMS(ESI-TOF) m / z calcd for C 21 h 24 N 3 [M+H] + :318.1965; found: 318.1967.
Embodiment 2
[0074]
[0075] Colorless oil; 28.7mg, 83% yield; column chromatography eluent, petroleummether / EtOAc=250:1; 1 H NMR (500MHz, CDCl 3 )δ7.45(d, J=7.8Hz, 1H), 7.02(d, J=7.9Hz, 1H), 6.98(t, J=7.4Hz, 1H), 6.93(t, J=7.3Hz, 1H) ,6.82(d,J=7.7Hz,1H),6.78-6.70(m,2H),6.68-6.61(m,1H),5.80-5.69(m,1H),5.65(t,J=7.5Hz,1H ),4.89(d,J=10.0Hz,1H),4.82(d,J=14.5Hz,1H),4.53(s,2H),4.38(d,J=17.5Hz,1H),4.13(d,J =14.0Hz,1H),3.41-3.31(m,1H),3.15-3.04(m,1H),2.85(s,3H),2.47-2,36(m,2H),2.20-2.02(m,2H ); 13 CNMR (125MHz, CDCl 3 )δ144.10,143.37,136.56,135.07,133.08,126.30,123.64,121.80,121.45,120.91,119.84,119.16,119.14,116.35,114.10,109.42,60.16,53.22,50.69,44.97,44.25,30.62,24.02;HRMS(ESI -TOF)m / zcalcd for C 23 h 26 N 3 [M+H] + :344.2121; found: 344.2122.
Embodiment 3
[0077]
[0078] Yellow solid; 35.0mg, 89% yield; mp 90-92°C; column chromatography yeluent, petroleum ether / EtOAc=200:1; 1 H NMR (500MHz, CDCl 3 )δ7.51-7.45(m,1H),7.12-7.05(m,3H),6.99(dt,J=6.2,3.3Hz,1H),6.97-6.92(m,2H),6.75(qd,J= 8.2,4.2Hz,2H),6.67(dd,J=10.0,4.5Hz,2H),6.63(dd,J=7.4,1.4Hz,2H),5.67(t,J=7.5Hz,1H),5.18( s,2H),4.85(d,J=14.0Hz,1H),4.12(d,J=14.0Hz,1H),3.43–3.32(m,1H),3.17-3.07(m,1H),2.69(s ,3H),2.48-2.40(m,2H),2.23-2.04(m,2H); 13 C NMR (125MHz, CDCl 3 )δ143.98,143.34,137.75,136.83,135.05,128.80(2C),127.17,126.38,125.86(2C),123.56,121.86,121.59,121.10,119.96,119.31,119.15,114.37,109.56,60.23,53.25,50.75,46.23 ,44.04,30.65,23.98; HRMS(ESI-TOF)m / z calcd for C 27 h 28 N 3 [M+H] + :394.2278; found: 394.2277.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com