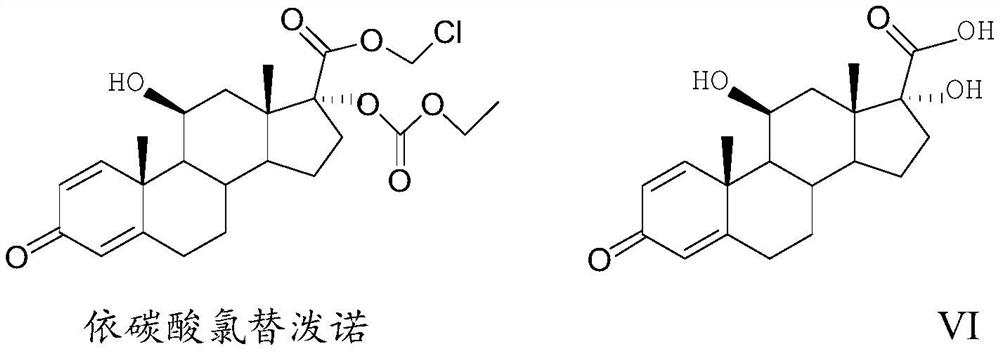
Preparation method of loteprednol etabonate intermediate
A technique of loteprednol etabonate and intermediates, applied in the field of preparation of loteprednol etabonate intermediates, can solve problems such as high cost of raw materials, long process routes, complicated operations, etc., and achieves low cost and shortened process routes. , the effect of simple operation
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
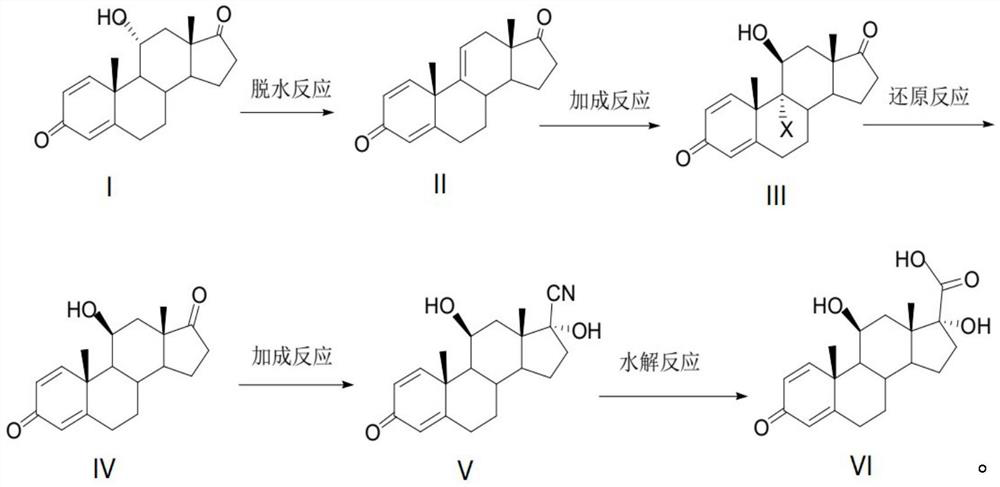
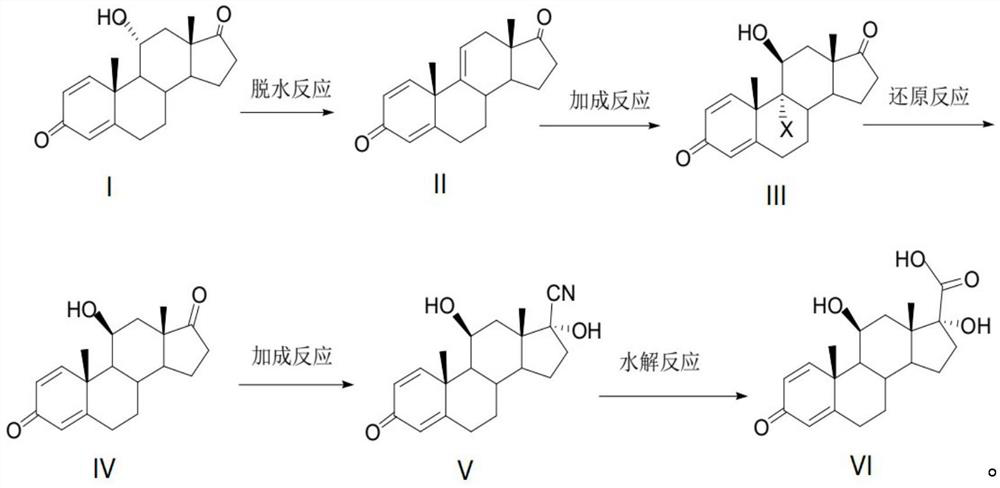
[0032] Add 250ml of tetrahydrofuran and 50g of 11α-hydroxyl-ADD (compound I) into the reaction flask under the protection of nitrogen. 10g, each interval of 20 minutes, control the temperature at -40°C after adding, react for 5h, then control the temperature at 10°C, slowly add 50ml of drinking water to stop the reaction, slowly add 150ml of 30% sodium hydroxide solution dropwise, adjust the pH of the system to Between 6 and 7, control the temperature at 25°C, let the layers stand for 30 minutes, wash the water layer with 100ml of tetrahydrofuran, stir for 10 minutes, let stand for 30 minutes to separate layers, combine the tetrahydrofuran layers, concentrate under reduced pressure, recover the solvent, and pour into 50ml of methanol Tetrahydrofuran was entrained, filtered, and dried for 10 hours to obtain 45.1 g of compound II with a mass yield of 90.2% and a purity of 98.5% as determined by high performance liquid chromatography.
[0033] Add 40g of compound II to 150ml of a...
Embodiment 2
[0038]Add 200ml of dichloromethane and 50g of compound I into the reaction flask under the protection of nitrogen, cool down to -50°C, add 50g of phosphorus pentachloride in 5 times, 10g each time, each time at an interval of 20 minutes, and control the temperature after adding - 40°C, react for 5 hours, then control the temperature at 10°C, slowly add 50ml of drinking water to stop the reaction, slowly add 150ml of 30% sodium hydroxide solution dropwise, adjust the pH of the system to 6-7, control the temperature at 25°C, and statically Separate the layers for 30 minutes, wash the water layer with 100 ml of dichloromethane, stir for 10 minutes, let stand for 30 minutes to separate layers, combine the dichloromethane layers, concentrate under reduced pressure, recover the solvent, pour into 50 ml of methanol to entrain the dichloromethane, filter, and dry for 10 42.5 g of compound II was obtained, with a mass yield of 85.0%, and a purity of 97.8% as determined by high performan...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com