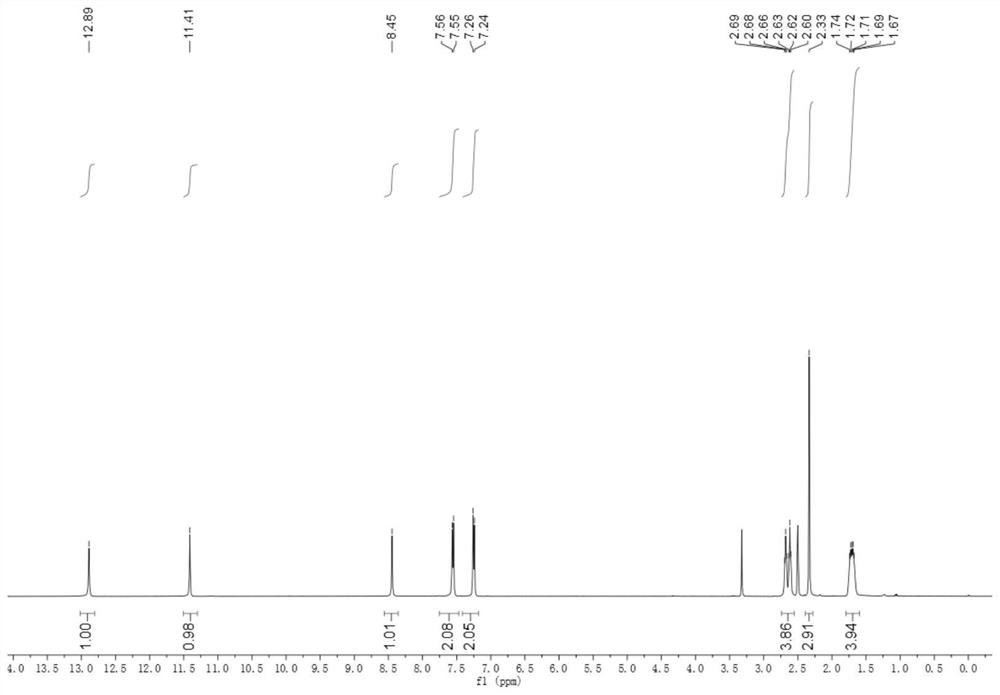
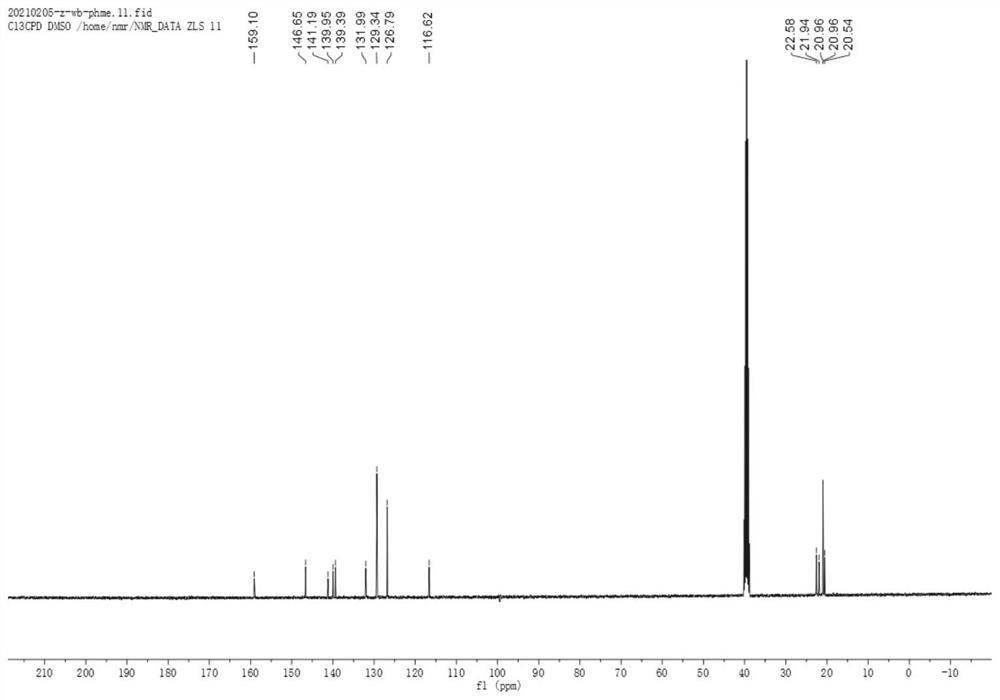
Application of indazole hydrazide compound in preparation of anti-tumor angiogenesis medicine
An indazole hydrazide, angiogenesis technology, applied in antitumor drugs, drug combinations, pharmaceutical formulations, etc., can solve problems such as drug resistance, and achieve the effect of inhibiting angiogenesis
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0064] Prepare the compound shown in formula (1) ~ formula (5) according to the following steps:
[0065]
[0066] Ph represents a substituted or unsubstituted phenyl group.
[0067] Sodium ethoxide solution was prepared by adding sodium (1.5 g, 65 mmol) to absolute ethanol (20 mL) at 0 °C; then a mixture of cyclohexanone (4.41 g, 44 mmol) and diethyl oxalate (7.3 g, 50 mmol) was added slowly , and stirred the solution at room temperature for 12 h, and then decomposed the reaction mixture with 2N sulfuric acid solution, extracted the mixture with ethyl acetate, dried and concentrated the organic solvent, and the obtained crude product was columnar with n-hexane:ethyl acetate (12:1) Further purification by chromatography gave yellow oil as product 2 (5.87 g, 67%).
[0068] Hydrazine (448 mg, 14 mmol) was slowly added to a cooled suspension of product 2 (2.38 g, 12 mmol) in acetic acid (5 mL), the mixture was heated to reflux for 1 h, poured into ice water, and washed with N...
Embodiment 2
[0093] Example 2 Determination of anti-tumor angiogenesis activity
[0094] 2.1 Evaluation of 2D angiogenesis experiments Anti-angiogenesis experiments of compounds:
[0095](1) In a 96-well plate, add 50ul of 100% Matrigel to each well to avoid air bubbles. Place in a 37°C incubator for 45 minutes.
[0096] (2) Digest well-growing HUVEC cells when their confluence reaches about 80%, and resuspend the cells with HUVEC complete medium. Add 40uL of resuspension solution to each well, the concentration is 4X10000 / well cells, repeat three wells.
[0097] (3) Add 5 uL of compounds with three concentrations of high, medium and low to the compound group to be tested. In the VEGF group, 5uL of culture solution containing 200ng / ml VEGF was added. The solvent group was supplemented with 10uL culture solution.
[0098] (4) Cultivate in a 5% CO2 incubator at 37°C for 4 hours, and take pictures of each well.
[0099] The result is as Figure 11 , Figure 12 shown.
[0100] The num...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com