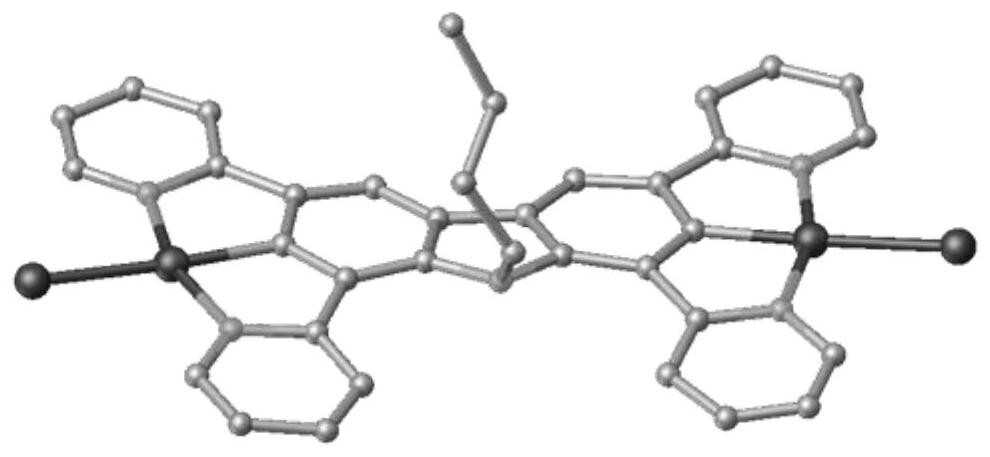
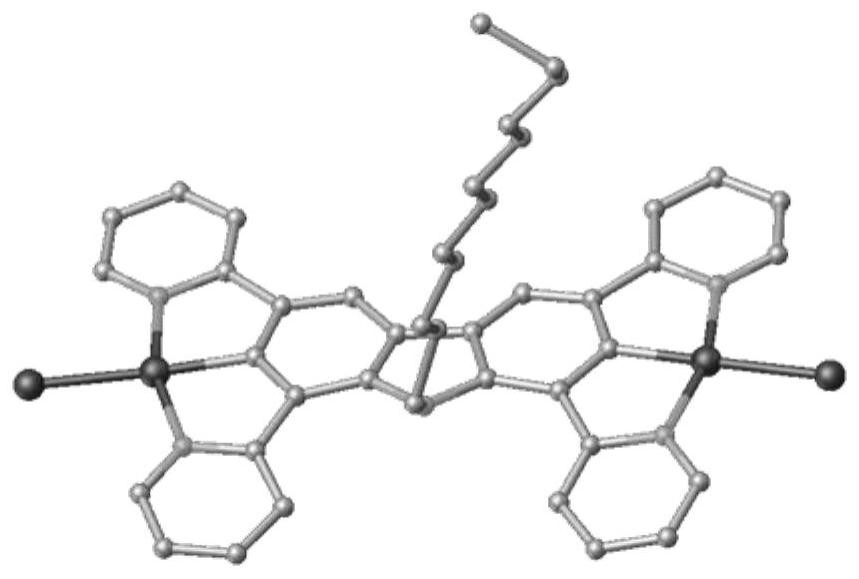
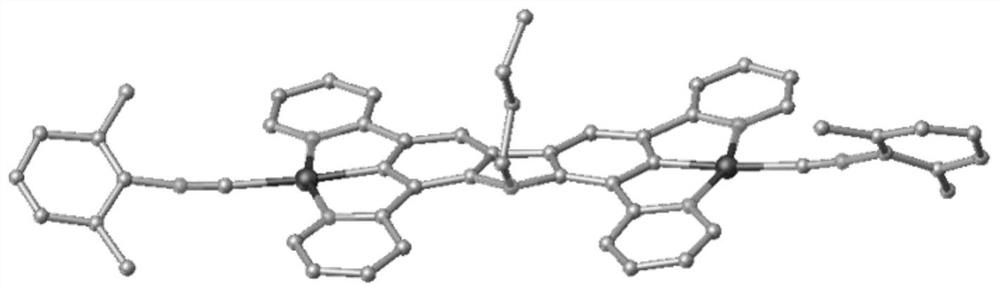
Carbazole bridged binuclear metal platinum complex as well as preparation method and application thereof
A technology of binuclear metal and platinum complexes, applied in platinum group organic compounds, platinum organic compounds, chemical instruments and methods, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0088] The embodiment of the present invention also provides a preparation method of a carbazole-bridged binuclear metal platinum complex, comprising:
[0089] Step 1, under nitrogen protection, 9-R group-1,3,6,8-tetrakis(pyridin-2-yl)-9H-carbazole and K 2 PtCl 4 Reflux reaction in acetic acid solution to obtain the first complex, the first complex is R 1 and R 2 It is a neutral carbazole bridged dinuclear metal platinum chloride complex (complex I-1 or complex I-2) with Cl and n being 0. After the reaction is completed, the reaction liquid is cooled, and suction filtered; the substance obtained by the suction filtration is washed.
[0090] Step 2. In KPF 6 In the presence of R 1 and R 2At least one of them is 2-isonitrile-1,3-xylene, and the other is an ionic carbazole-bridged binuclear metal platinum complex of Cl or 2-isonitrile-1,3-xylene. After the reaction is completed, the reaction solution is sequentially extracted, dried, filtered, separated and purified to obt...
Embodiment 1
[0114] The synthesis of 9-butyl-1,3,6,8-tetrakis(pyridin-2-yl)-9H-carbazole refers to Wang L, Yang W-W, ZhongY-W, Yao J.Enhancing the Electronic Coupling in Cyclometalated BisrutheniumComplex by Using the 1,3,6,8-Tetra-(pyridin-2-yl)carbazole Bridge[J].DaltonTrans.,2013,42,5611-5614. The prepared compound 9-butyl-1,3,6,8-tetrakis(pyridin-2-yl)-9H-carbazole (54mg, 0.1mol), K 2 PtCl 4 (91mg, 0.22mol) was added to a 50mL two-necked flask containing 7mL of acetic acid under nitrogen protection, heated to 120°C, and reacted for three days. After the reaction was completed, cool to room temperature, precipitated solid, and suction filtered. The obtained solid was washed successively with water (50 mL), methanol (20 mL), and ether (50 mL) to remove some impurities. Then, wash with about 100 mL of dichloromethane solvent until the filtrate is light yellow-green, collect the obtained organic filtrate, and concentrate to dryness to obtain a pure orange-red solid, yield: 53%.
[0115]...
Embodiment 2
[0119] The synthesis of 9-dodecyl-1,3,6,8-tetrakis(pyridin-2-yl)-9H-carbazole refers to Wang L, Yang W-W, Zhong Y-W, Yao J. Enhancing the Electronic Coupling in Cyclometalated Bisruthenium Complex by Using the 1,3,6,8-Tetra-(pyridin-2-yl)carbazoleBridge [J]. Dalton Trans., 2013, 42, 5611-5614. The prepared compound 9-dodecyl-1,3,6,8-tetrakis(pyridin-2-yl)-9H-carbazole (64mg, 0.1mol), K 2 PtCl 4 (91mg, 0.22mol) was added into a 50mL two-necked flask containing 9mL of acetic acid under nitrogen protection, heated to 120°C, and reacted for three days. After the reaction was completed, cool to room temperature, precipitated solids, and suction-filtered. The obtained solids were washed with water (50 mL), methanol (20 mL), and ether (50 mL) in sequence to remove some impurities. Then, it was washed with about 100 mL of dichloromethane solvent until the filtrate was light yellow-green, and the obtained organic filtrate was collected and concentrated to dryness to obtain a pure ora...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com