Application of halcinonide and derivative thereof in preparation of medicine for treating and/or preventing cerebrovascular diseases
A technology for cerebrovascular diseases and derivatives, applied in the field of medicine, can solve the problems of cerebrovascular diseases without the application of hacinide
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
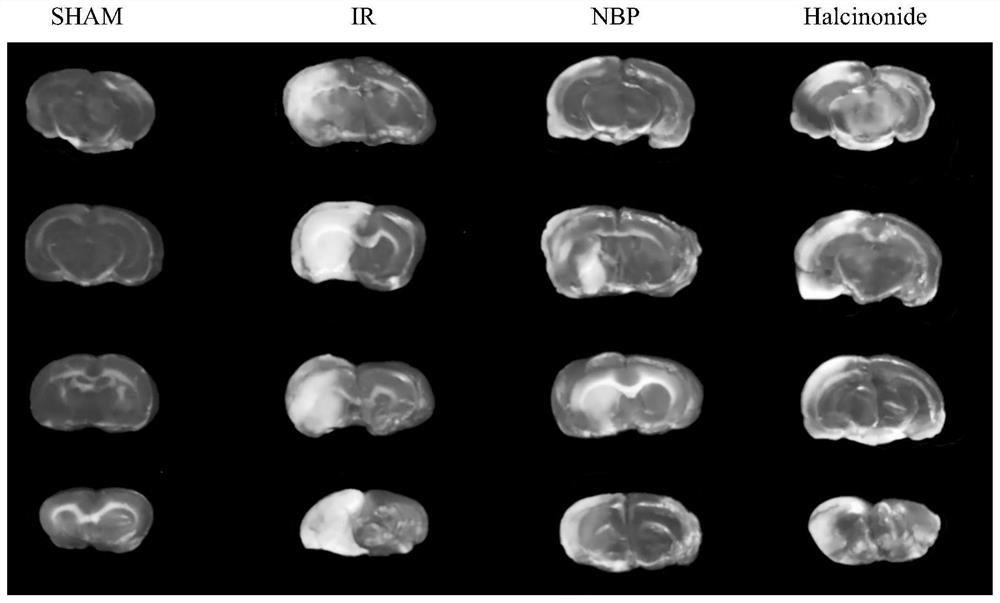
[0069] Example 1. Study on the Effect of Halcinonide in Treating Ischemic Stroke
[0070] 1. Experimental animals
[0071] 3-month-old SPF male SD rats were selected, with a body weight of 200±20 g. Provided by Lanzhou Veterinary Research Institute, Chinese Academy of Agricultural Sciences. No drugs were used before the experiment. The experimental animals were fed for one week in an environment with a temperature of 24-26°C and a 12h / 12h regular alternation of daylight hours, and the animals were given food and water freely, and then the experiments were performed in groups.
[0072] 2. Drugs and reagents
[0073] Halcinonide, purchased from Shanghai Macklin Biochemical Technology Co., Ltd., batch number: C11650940, molecular weight formula C 24 h 32 ClFO 5 , with a molecular weight of 454.96
[0074]Butylphthalide soft capsules (Butylphthalide, NBP), purchased from CSPC NBP Pharmaceutical Co., Ltd.;
[0075] 2,3,5-Triphenyltetrazolium chloride (TTC) was purchased fro...
Embodiment 2
[0101] Example 2. Study on the Effect of Prednisone in Treating Ischemic Stroke
[0102] 1. The specific implementation method is the same as embodiment one.
[0103] Prednisone (Prednisone) was purchased from Shanghai Macklin Biochemical Technology Co., Ltd., batch number: C11008416, and the molecular formula is C 21 h 26 o 5 , the molecular weight is 358.43. For the experimental grouping, see Example 1. The halcinonide group was replaced by the prednisone group. The administration method was gavage, and the dose was 20 mg / kg / d. Model preparation and detection indicators are the same as in Example 1.
[0104] 2. Experimental results
[0105] (1) Assessment of neurological deficit score
[0106] SD rats were administered for 1 day, and after 2 hours of ischemia in the MCAO model, they were treated with drugs again, and after 24 hours of reperfusion, the neurofunctional and behavioral scores of rats in each group were shown in the appendix. Figure 4 . Such as Figure ...
Embodiment 3
[0109] Example 3. Study on the Effect of Testosterone Propionate in Treating Ischemic Stroke
[0110] 1. Refer to Embodiment 1 for the specific implementation method.
[0111] Testosterone Propionate (Testosterone Propionate) was purchased from Shanghai Aladdin Biochemical Technology Co., Ltd., batch number: A1910098, and the molecular formula is C 22 h 32 o 3 , the molecular weight is 344.49. For the experimental grouping, see Example 1. The halcinonide group was replaced by the testosterone propionate group. The administration method was gavage, and the dose was 20 mg / kg / d. Model preparation and detection indicators are the same as in Example 1.
[0112] 2. Experimental results
[0113] (1) Assessment of neurological deficit score
[0114] SD rats were administered for 1 day, and after 2 hours of ischemia in the MCAO model, they were treated with drugs again, and after 24 hours of reperfusion, the neurofunctional and behavioral scores of rats in each group were shown i...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com