Cyclopentane coumarin compound and preparation method thereof
A technology for coumarins and compounds is applied in the field of cyclopentane coumarin compounds and their preparation, and achieves the effects of fast reaction speed, easy separation and purification, and rich diversity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
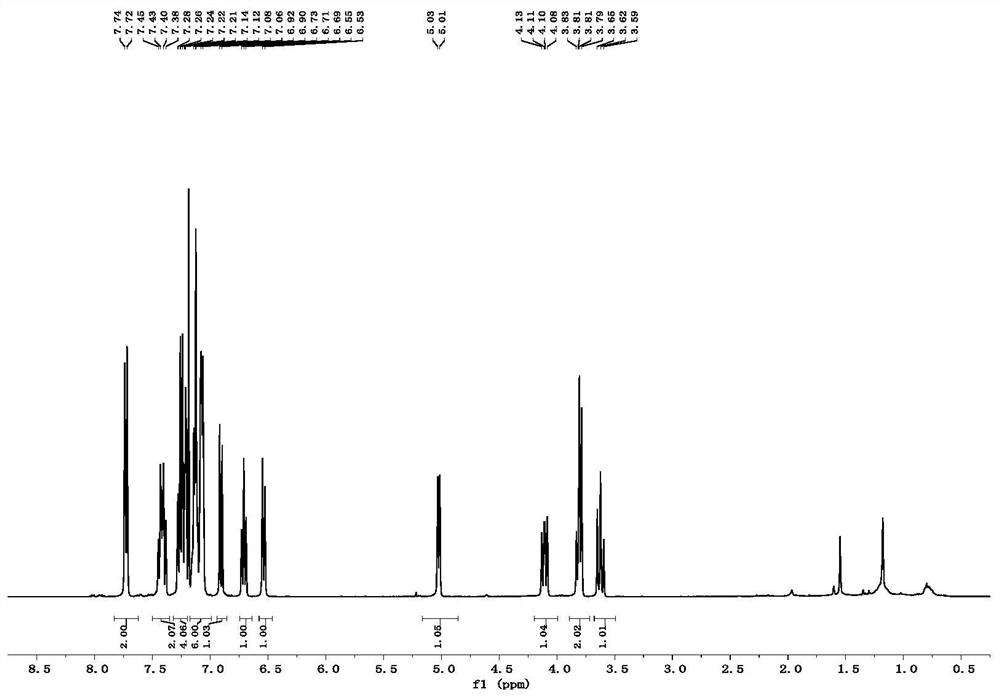
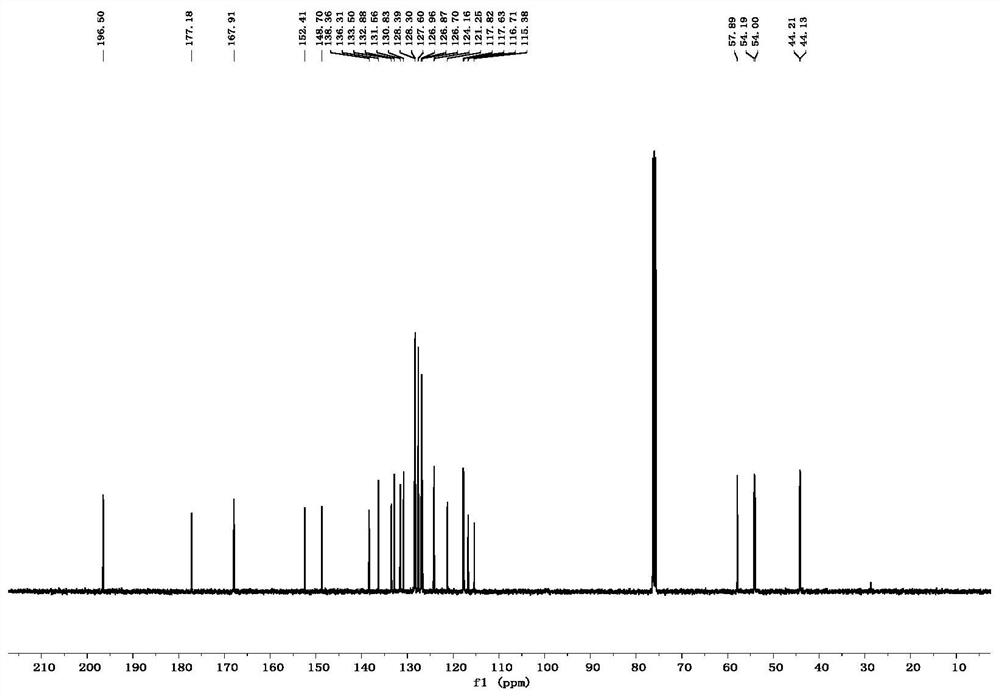
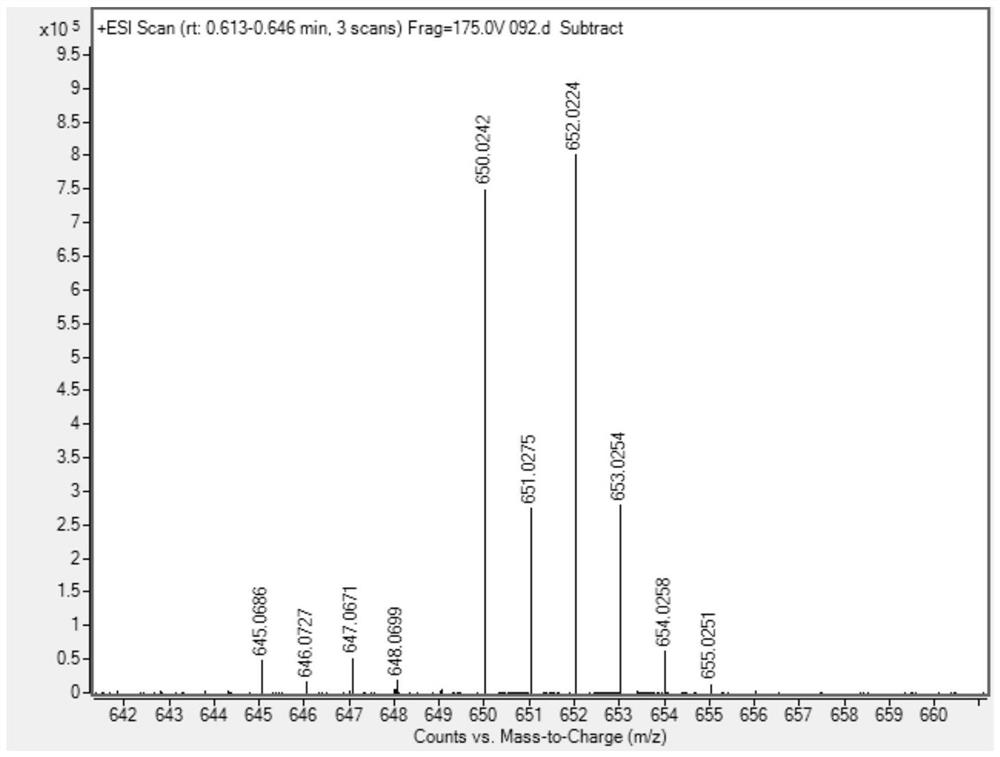
[0049] Put 28.5mg (0.1mmol) of compound 1a, 41.2mg (0.12mmol) of compound 2a and 1mL of tetrahydrofuran into a dry 15mL Shrek tube, add 3.0mg (0.02mmol) of 1,8-diazabicyclonedecyl Carb-7-ene mixed for cycloaddition reaction. In the reaction system, the molar ratio of Compound 1a to Compound 2a is 1:1.2, 1,8-diazabicycloundec-7-ene accounts for 20% of the molar percentage of Compound 1a, and stirred at 25°C After 12 hours, it was concentrated with a rotary evaporator, and purified through a silica gel column (dichloromethane:petroleum ether=2:1, v / v) to obtain 54.0 mg of product 3aa with a yield of 85%.
[0050]
Embodiment 2
[0052]Put 28.5mg (0.1mmol) of compound 1a, 41.2mg (0.12mmol) of compound 2a and 1mL of tetrahydrofuran into a dry 15mL Shrek tube, add 2.2mg (0.02mmol) of triethylenediamine (DABCO) and mix well for ring Addition reaction. In the reaction system, the molar ratio of compound 1a to compound 2a is 1:1.2, triethylenediamine (DABCO) accounts for 20% of the molar percentage of compound 1a, stirred at 25°C for 12 hours, and concentrated with a rotary evaporator, Purified on a silica gel column (dichloromethane:petroleum ether=2:1, v / v) to obtain 35.8 mg of product 3aa with a yield of 57%.
[0053]
[0054] Compared with Example 1, the catalyst is replaced by DABCO, and others are the same as Example 1.
Embodiment 3
[0056] Put 28.5mg (0.1mmol) of compound 1a, 41.2mg (0.12mmol) of compound 2a and 1mL of tetrahydrofuran into a dry 15mL Shrek tube, add 2.4mg (0.02mmol) of 4-dimethylaminopyridine (DMAP) and mix well Carry out a cycloaddition reaction. In the reaction system, the molar ratio of compound 1a to compound 2a is 1:1.2, 4-dimethylaminopyridine (DMAP) accounts for 20% of the molar percentage of compound 1a, stirred at 25° C. for 12 hours, and Concentrate and purify through a silica gel column (dichloromethane:petroleum ether=2:1, v / v) to obtain 39.0 mg of product 3aa with a yield of 62%.
[0057]
[0058] Compared with Example 1, the catalyst is replaced by DMAP, and others are the same as Example 1.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com