Morpholine-pyridine-merocyanine derivative fluorescent probe as well as preparation method and application thereof
A technology of fluorescent probes and derivatives, applied in the direction of fluorescence/phosphorescence, chemical instruments and methods, luminescent materials, etc., can solve the problems of uncommon CEs ratio fluorescent probes, achieve wide application value, easy access to raw materials, synthesis and The effect of simple post-processing methods
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0030] The preparation method of the morpholine-pyridine-merocyanine derivative fluorescent probe of this embodiment is as follows:
[0031] 4.41 g 4-[2-(6-hydroxy-2-naphthyl)-vinyl]-1-[2-(4-morpholinoethyl)]-pyridinium bromide (10 mmol) and 1.23 g ethyl Acid anhydride (12 mmol) was dissolved in 0.2 L of dichloromethane, 3.04 g of triethylamine (30 mmol) was added dropwise as a catalyst, stirred at room temperature for 3-4 h, after the reaction was traced by TLC, rotary evaporation was performed, and the obtained solid was washed with ethyl acetate The ester was washed, and then recrystallized with absolute ethanol to obtain the fluorescent probe of the morpholine-pyridine-merocyanine derivative. The yield of the target product was 65%.
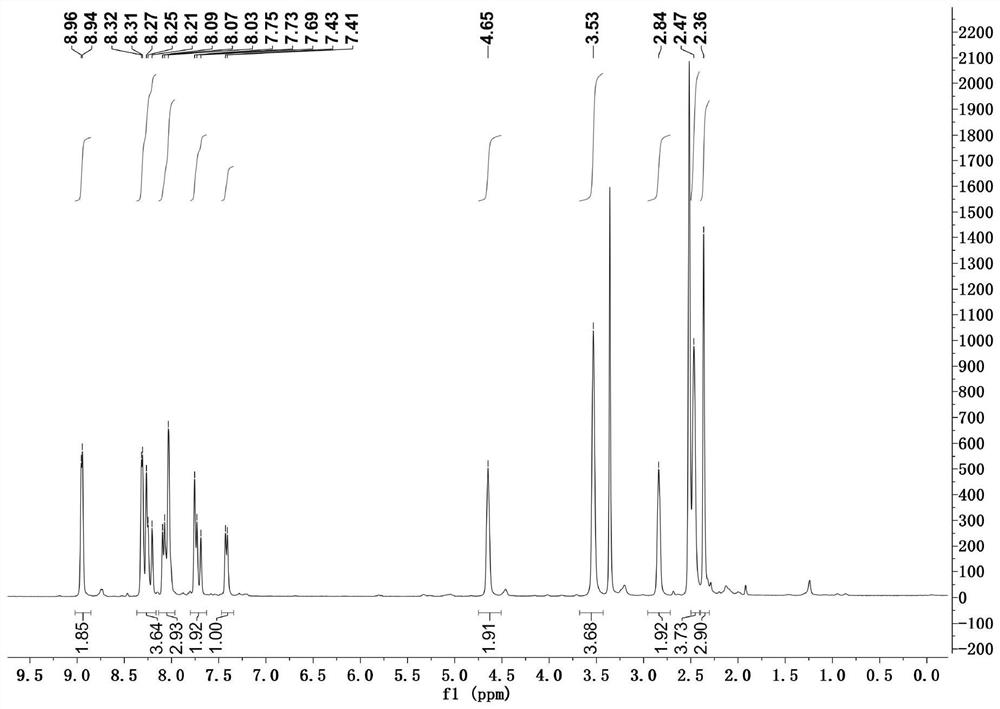
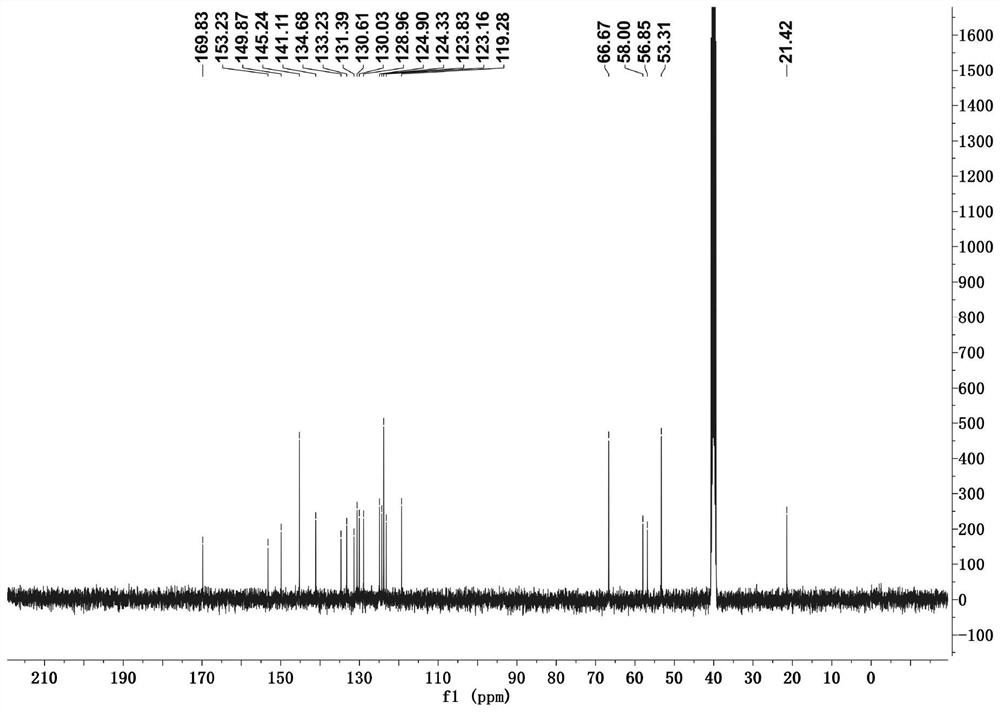
[0032] Adopt nuclear magnetic resonance instrument to carry out nuclear magnetic resonance analysis to the morpholine-pyridine-merocyanine derivative that makes, the result is as follows:
[0033] 1 H NMR (400 MHz, DMSO- d 6 ), δ (ppm): ...
Embodiment 2
[0037] Determination of Optical Properties of Morpholine-Pyridine-Merocyanine Derivatives on CEs
[0038] The morpholine-pyridine-merocyanine derivatives prepared in the above-mentioned Example 1 were used as fluorescent probes in PBS sodium buffer solution (0.01 mol / L, pH=7.4) to prepare a molar concentration of 1×10 -5 mol / L solution, respectively, at a molar concentration of 2×10 -5 mol / L anion (AcO − , ClO − , CO 3 2− , S 2− and HCO 3 − ), cations (Ca 2+ , Mg 2+ 、Al 3+ and Fe 3+ ) reactive oxygen species (H 2 o 2 ) and amino acids (Cys, Ala, Ary, Ser, Asp) were added with an equal amount of the above fluorescent probe solution, and analyzed with a UV-Vis spectrophotometer or a fluorescence spectrometer (excitation wavelength is 360 nm), and the obtained UV and fluorescence Spectrum see Figure 4 . pass Figure 4 It can be seen that the morpholine-pyridine-merocyanine derivatives prepared in the present invention have obvious responses only to CEs as pro...
Embodiment 3
[0041] Detection experiment of morpholine-pyridine-merocyanine derivative fluorescent probe in intracellular CEs
[0042] 1 x 10 for HeLa cells -5 mol / L of the morpholine-pyridine-merocyanine derivative fluorescent probe prepared in Example 1 above was incubated at 37°C for 30 minutes, and CEs were added to ensure that the concentration of CEs was 1×10 -3 U / L, and then cultivated for 30 minutes to obtain the fluorescence imaging map of HeLa cells, specifically as follows Image 6 As shown, where: a is the fluorescence imaging image of the green channel of the fluorescent probe; b is the fluorescence imaging image of the red channel of the fluorescent probe; c is the overlay image of the green channel and the red channel of the fluorescent probe; d is the bright field image of the fluorescent probe; e The green channel, red channel and bright field overlay of the fluorescent probe; f is the fluorescence imaging image of the green channel after the fluorescent probe + CEs; g t...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com