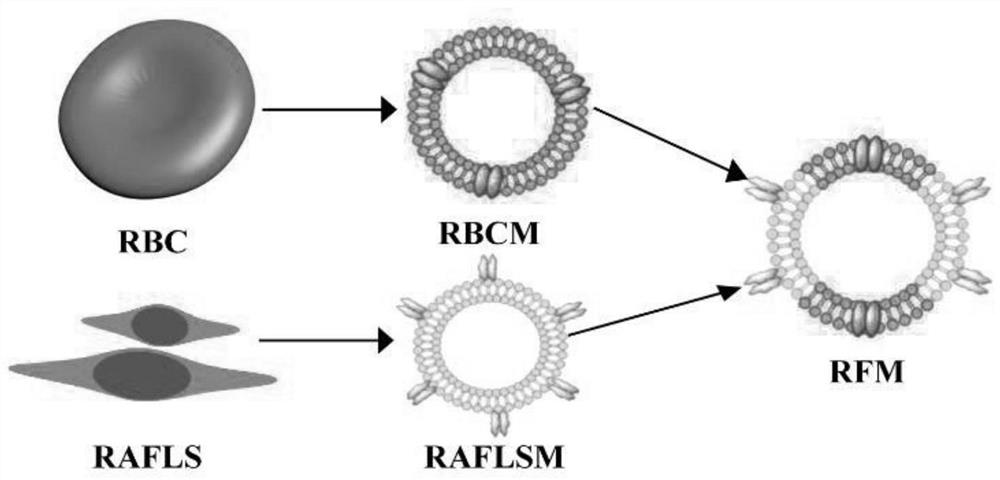
Cytokinin targeted drug-carrying system, preparation method and application thereof
A drug-loading and targeting technology, applied in the preparation of anti-rheumatoid arthritis drugs, preparation of hemocytin-targeted drug-loading system, field of hemocytin-targeted drug-loading system, can solve the problem of rheumatoid arthritis The effect of long-term treatment of rheumatoid arthritis, rheumatoid arthritis can not be cured, reduce patient tolerance and other problems, to achieve the effect of reducing adverse reactions, reducing the concentration of hemocyclin, and increasing the concentration of hemocyclin
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0037] 1. Synthesis of Prussian blue nanoparticles:
[0038] Take 0.016 g of potassium ferricyanide and 2 g of PVP, successively add them to 40 mL of 0.01 M HCl, and stir to dissolve for 30 min at room temperature. The yellow clear solution obtained after dissolving was placed in an oil bath at 80° C. for static reaction for 20 hours to obtain crude PB nanomaterials.
[0039] The crude PB nanomaterials were dispensed into 1.5 mL EP tubes, and centrifuged at 13500 rpm for 15 minutes at 4°C. After centrifugation, discard the supernatant, combine the materials in each two EP tubes into one tube, add 1 mL of sterile deionized water, mix thoroughly, centrifuge under the same conditions for 15 minutes, and then add 1 mL of sterile deionized water to each tube. The EP tube was concentrated into 1 tube to obtain refined PB material.
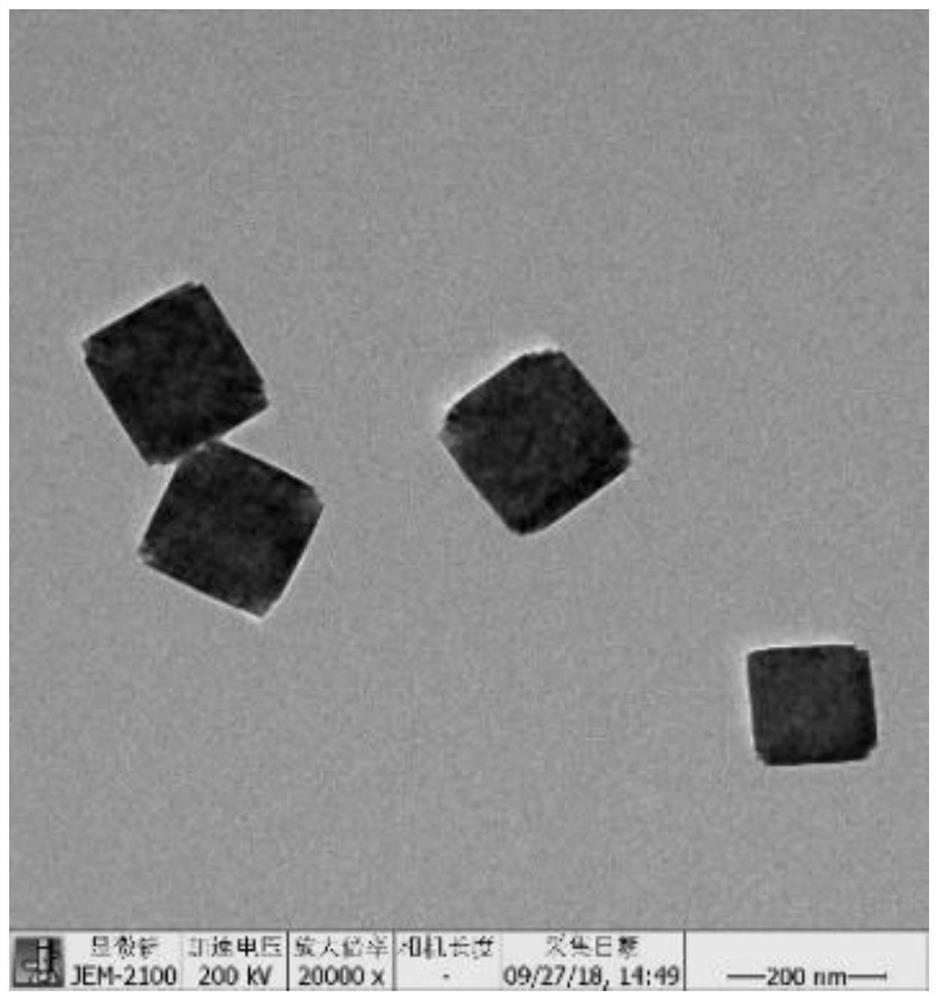
[0040] Take 5 μL of 0.05 mg / mL refined PB material, drop it on the cut silicon wafer, place it in an oven to dry at 45°C, and photograph it with a sca...
Embodiment 2
[0059] Take 20 μL of HA@RFM@PB@SE NPs (0.01mg / mL) solution and drop it on the copper mesh covered with carbon film, put it under infrared lamp and dry it at 60℃, use transmission electron microscope to photograph its shape and RFM film Status of the package. like Figure 5 As shown, the HA@RFM@PB@SE NPs displayed a typical core-shell structure.
[0060] 100 μL each of PB NPs, PB@SE NPs, RFM@PB@SE NPs and HA@RFM@PB@SE NPs were placed in the sample cuvette, and their particle size distribution and Zeta were measured by Zeta potential and nanoparticle size analyzers, respectively. potential. structure as Image 6 , Figure 7 shown. like Image 6 As shown, the peak particle size of PB NPs and PB@SE NPs are both around 80 nm, while the particle size distribution of PB@SE NPs is wider, and there are more large-sized particles, indicating that the particle size of PB nanoparticles does not change after loading SE. large, and some particles have increased. The particle size of...
Embodiment 3
[0062] Whole blood samples of SPF adult SD rats were taken, centrifuged at 3000 rpm for 5 min at 4°C, and washed 5 times with PBS to obtain pure red blood cells. 50 μL of 4% red blood cells (v / v) and 950 μL of PB NPs, PB at different concentrations (12.5 μg / mL, 25 μg / mL, 50 μg / mL, 100 μg / mL, and 200 μg / mL, respectively, dispersant in PBS) The @SE NPs, RFM@PB@SE NPs, and HA@RFM@PB@SE NPs were mixed, and the mixture was allowed to stand at 37 °C for 4 hours. The positive control was mixed with pure water and red blood cells, and the red blood cells were 100% hemolyzed after mixing. The mixture was centrifuged at 3000 rpm for 5 minutes at 4°C, and the absorbance of the supernatant at 540 nm was measured using a UV-Vis spectrophotometer. By formula: Hemolysis(%)=(I / I 0)×100% to calculate the hemolysis rate, in the formula, Hemolysis is the hemolysis rate, I represents the absorbance of the detected sample, I 0 Indicates the absorbance of the positive control (100% hemolysis). ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle diameter | aaaaa | aaaaa |
| particle diameter | aaaaa | aaaaa |
| particle diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com