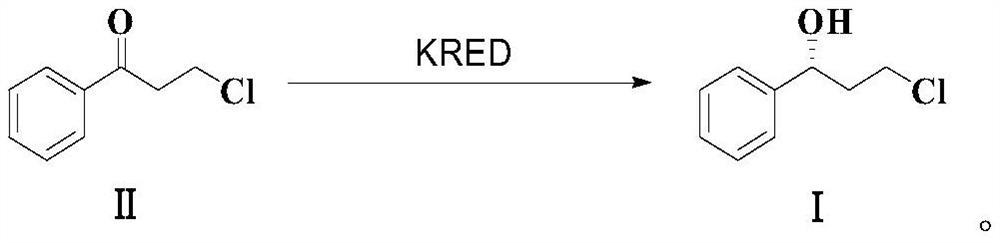
Enzymatic synthesis method of (R)-3-chloro-1-phenyl-propan-1-ol
A technology of chlorophenylpropanol and synthetic method, applied in the field of biopharmaceuticals, to achieve good industrialization potential, excellent stereoselectivity, and environmental friendliness
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0027] Step 1: Preparation of Ketocarbonyl Reductase Genetic Engineering Bacteria
[0028] The ketocarbonyl reductase is derived from a wild yeast strain (Candida ATCC2001), and its nucleotide sequence is shown in SEQ ID No.3.
[0029] After the nucleotide sequence of ketone carbonyl reductase from wild yeast was amplified by error-prone PCR, it was loaded into the expression plasmid pRSFDuet-1, the double restriction sites were HindⅢ and BamHI, and the primers were:
[0030] F: CGCGGATCCATGGCTGCTCTACATAAGAACA and
[0031] R: CCCAAGCTTTTACACAAATGGCTTAAATGGCC,
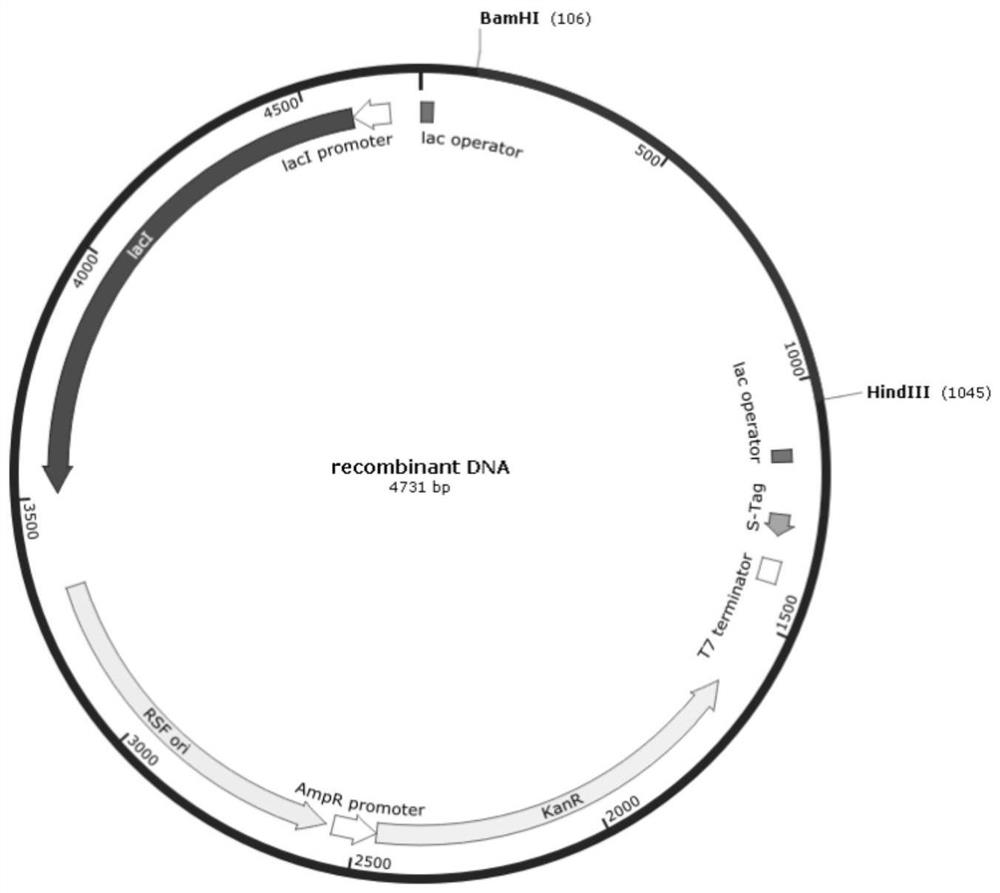
[0032] The recombinant expression plasmid ( figure 1 ) into E.coli BL21 (DE3) competent, pick positive transformants and sequence their nucleotide sequence as shown in SEQ ID No.1 (optimized carbonyl reductase DNA sequence) to obtain ketone carbonyl reductase Expression engineering bacteria: TM-01.
[0033] Step 2: Preparation of recombinant ketone carbonyl reductase
[0034] The obtained carbonyl reductase enginee...
Embodiment 2
[0035] Embodiment 2: the preparation of (R)-3-chlorophenylpropanol
[0036] In the 250ml Erlenmeyer flask, add 60ml PB damping fluid (0.2mM, pH6.5), dissolve successively the recombinant ketone carbonyl reductase that 800mg embodiment 1 obtains, 200mg isopropanol dehydrogenase enzyme powder (commercial enzyme, product number WT2302, purchasing company: Alpha Technology), 10mg NADP+ / NADPH. 1g of the substrate 3-chloropropiophenone was dissolved in 40ml of isopropanol, added to the reactor, stirred at 200rpm, and reacted at 25°C for 16h to obtain (R)-3-chloropropiophenone. The reaction result was detected by HPLC, the conversion rate was 90%, the purity was 99.68%, and the ee value was 99.9%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com