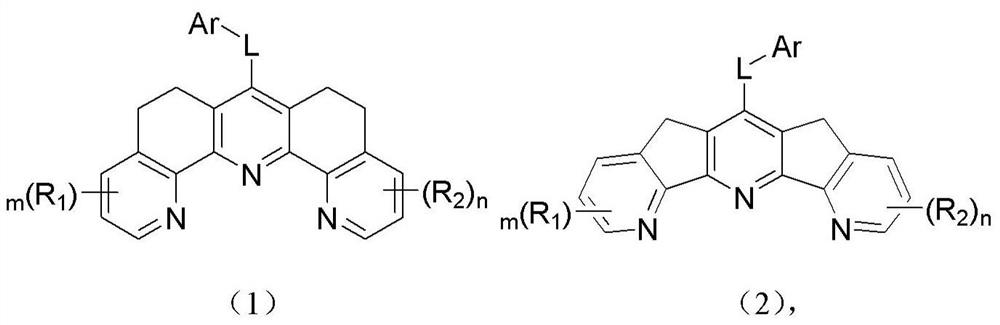
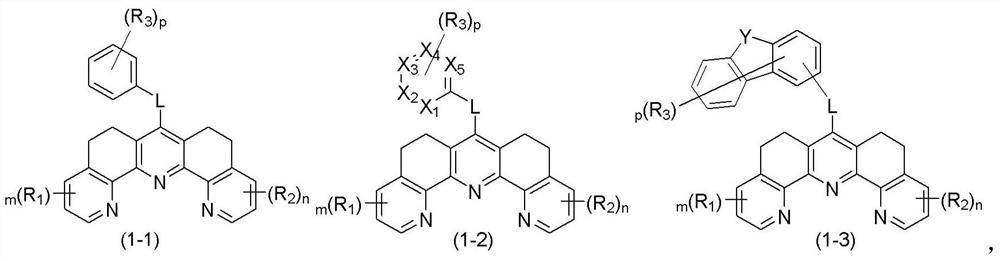
Organic compound and organic electroluminescent device containing same
An organic compound and organic technology, applied in the field of new organic compounds, to achieve the effects of good electron injection and migration performance, strong metal coordination ability, and high external quantum efficiency
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Synthetic example 1
[0060] Synthesis Example 1: Synthesis of Compound C1
[0061]
[0062] (1) Preparation of compound 1-1
[0063] 6,7-dihydro-5H-quinolin-8-one (147g, 1mol), morpholine (174g, 2mol), p-toluenesulfonic acid (3.5g, 0.02mol, 3eq) were added to a solution containing toluene (1.5L ) in a three-neck flask. The oil bath was heated to reflux, the water separator reacted for 20 hours, and the reaction was completed by TLC monitoring. The reaction solution was cooled to room temperature, and the solvent was removed by rotary evaporation under reduced pressure. The obtained crude product was purified by column chromatography to obtain intermediate 1-1 (119 g, yield 55%).
[0064] (2) Preparation of compound 1-2
[0065] Add intermediate 1-1 (108g, 0.5mol) and p-chlorobenzaldehyde (42g, 0.3mol) into a three-necked flask containing 1,4-dioxane (1L). The oil bath was heated to reflux for 25 hours, and the reaction was monitored by TLC. The reaction solution was cooled to room tempera...
Synthetic example 2
[0070] Synthesis Example 2: Synthesis of Compound C6
[0071]
[0072] (1) Preparation of compound C6
[0073] Compound 1-3 (8.8g, 18mmol), compound 4-chloro-2,6-diphenylpyrimidine (4.8g, 18mmol), potassium carbonate (7.45g, 54mmol), pd(PPh 3 ) 4 (208 mg, 0.18 mmol) was added into a flask containing 100 mL of toluene, 25 mL of ethanol and 25 mL of water, nitrogen was replaced and heated under reflux for 3 hours in a nitrogen atmosphere. TLC showed that the reaction was complete. The precipitated solid was filtered, rinsed with water and ethanol respectively, dried and purified by column chromatography to obtain compound C6 (8.8 g, yield 83%). Molecular weight calculated: 591.24, found C / Z: 591.2.
Synthetic example 3
[0074] Synthesis Example 3: Synthesis of Compound C45
[0075]
[0076] (1) Preparation of compound 3-1
[0077] 2-Phenyl-6,7-dihydro-5H-quinolin-8-one (223g, 1mol), morpholine (174g, 2mol), p-toluenesulfonic acid (3.5g, 0.02mol, 3eq) were added to In a three-necked flask containing toluene (1.5L). The oil bath was heated to reflux, and the water separator reacted for 25 hours, and the reaction was completed by TLC monitoring. The reaction solution was cooled to room temperature, and the solvent was removed by rotary evaporation under reduced pressure. The obtained crude product was purified by column chromatography to obtain intermediate 3-1 (149 g, yield 51%).
[0078] (2) Preparation of compound 3-2
[0079] Add intermediate 3-1 (146g, 0.5mol) and p-chlorobenzaldehyde (42g, 0.3mol) into a three-necked flask containing 1,4-dioxane (1L). The oil bath was heated to reflux for 22 hours, and the reaction was monitored by TLC. The reaction solution was cooled to room tem...
PUM
| Property | Measurement | Unit |
|---|---|---|
| current efficiency | aaaaa | aaaaa |
| external quantum efficiency | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com