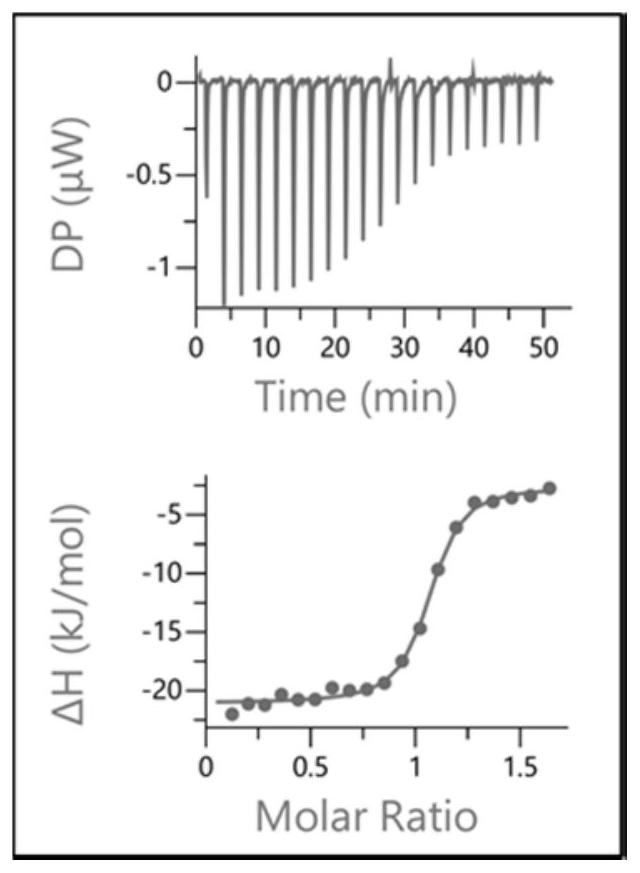
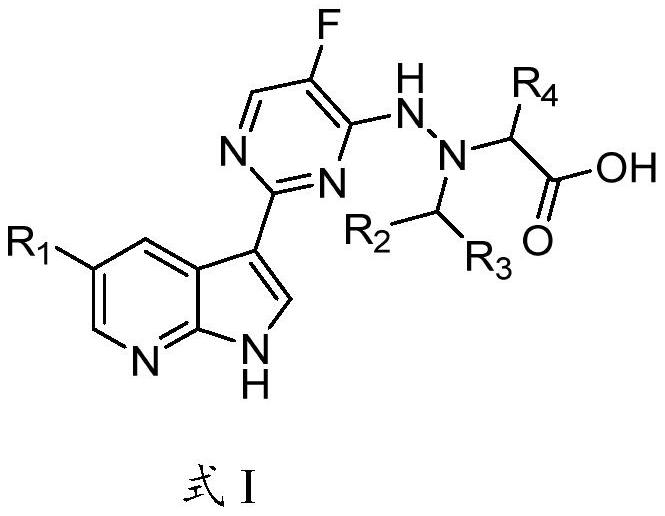
Azaindole derivative containing aza amino acid as well as preparation and application thereof
A technology of heteroindole derivatives and amino acids, which is applied in the field of azaindole derivatives, can solve the problems of lack of influenza virus, and achieve great application value, good inhibitory effect, and low toxicity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0039] Example 1 N-((5-fluoro-2-(1H-pyrrolo[2,3-b]pyridin-3-yl)pyrimidin-4-yl)amino)-N-neopentylglycine (Aza-01) ;
[0040] step:
[0041] Starting material 1: 3-Bromo-1-toluenesulfonyl-1H-pyrrolo[2,3-b]pyridine
[0042]
[0043] 3-Bromo-1H-pyrrolo[2,3-b]pyridine (10mmol) was dissolved in DMF, and NaH (15mmol) was added in batches under ice-cooling. After reacting for 30min, p-toluenesulfonyl chloride was added. TLC monitoring, after the disappearance of the raw materials, add water to the system to stop the reaction, a large amount of solid precipitated, continue stirring at room temperature for 30 min to completely precipitate the solid. A flesh-pink solid was obtained by suction filtration with a yield of 93%.
[0044] 1 H NMR (500MHz, CDCl 3 )δ8.33(dd, J=5.0,1.5Hz,1H),7.93(d,J=8.5Hz,2H),7.67-7.64(m,2H),7.15(s,1H),7.13-7.10(m ,2H), 2.23(s,3H).
[0045] Raw material 2: Preparation of tert-butyl 2-(2,2-dimethylpropylene)hydrazine-1-carboxylate:
[0046]
[0047]...
Embodiment 2
[0069] Example 2N-cyclopentyl-N-((5-fluoro-2-(1H-pyrrolo[2,3-b]pyridin-3-yl)pyrimidin-4-yl)amino)glycine (Aza-03)
[0070] Referring to the method of Example 1, except that N-((5-fluoro-2-(1-toluenesulfonyl-1H-pyrrolo[2,3-b]pyridin-3-yl)pyrimidin-4-yl)amino )-N-Neopentylglycine ethyl ester was replaced by N-cyclopentyl-N-((5-fluoro-2-(1-tosyl-1H-pyrrolo[2,3-b]pyridine-3- base) pyrimidin-4-yl) amino) glycine ethyl ester. White solid, 49% yield.
[0071] m.p.:>250℃; 1 H NMR (500MHz, DMSO-d 6 )δ12.13(s,1H),9.66(s,1H),8.9(d,J=7.5Hz,1H),8.25(dd,J=5.0,2.0Hz,1H),8.16(d,J=4.0 Hz,1H),8.10(s,1H),7.17-7.14(m,1H),3.70-3.65(m,1H),3.37(s,2H),1.67-1.65(m,6H),1.50-1.48( m,2H). 13 C NMR (125MHz, DMSO-d 6 )δ173.9, 158.5(d, J=6.25Hz), 152.1(d, J=7.50Hz), 149.1, 143.1, 142.1(d, J=261.25Hz), 130.4, 128.2(d, J=3.75Hz), 125.6 ,118.3,116.6,113.6,65.7,58.3,30.8,23.8.HRMS(ESI):m / z calcd for(C 18 h 19 FN 6 o 2 +H) + :371.1626; found: 371.1623.
Embodiment 3
[0072] Example 3 N-cyclohexyl-N-((5-fluoro-2-(1H-pyrrolo[2,3-b]pyridin-3-yl)pyrimidin-4-yl)amino)glycine (Aza-04)
[0073] Referring to the method of Example 1, except that N-((5-fluoro-2-(1-toluenesulfonyl-1H-pyrrolo[2,3-b]pyridin-3-yl)pyrimidin-4-yl)amino )-N-Neopentylglycine ethyl ester was replaced by N-cyclohexyl-N-((5-fluoro-2-(1-tosyl-1H-pyrrolo[2,3-b]pyridin-3-yl ) pyrimidin-4-yl) amino) glycine ethyl ester. White solid, yield 51%.
[0074] m.p.:>205.5-206.9℃; 1 H NMR (500MHz, DMSO-d 6 )δ12.09(s,1H),8.69(dd,J=8.0,1.5Hz,1H),8.27(dd,J=4.5,1.5Hz,1H),8.17-8.16(m,2H),7.20-7.17 (m,1H),4.79(s,2H),4.35–4.27(m,1H),1.86-1.82(m,2H),1.80-1.73(m,4H),1.68-1.66(m,1H),1.44 –1.37(m,2H),1.17-1.17(m,1H). 13 C NMR (125MHz, DMSO-d 6 )δ157.5(d, J=5.00Hz), 152.4(d, J=1.25Hz), 149.1, 143.3(d, J=253.75Hz), 143.1, 129.6, 128.7, 125.5, 118.0, 116.6, 113.5, 57.4 ,49.4,32.1,28.9,25.3.HRMS(ESI):m / z calcd for(C 19 h 21 FN 6 o 2 +H) + :385.1783; found: 385.1779.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com