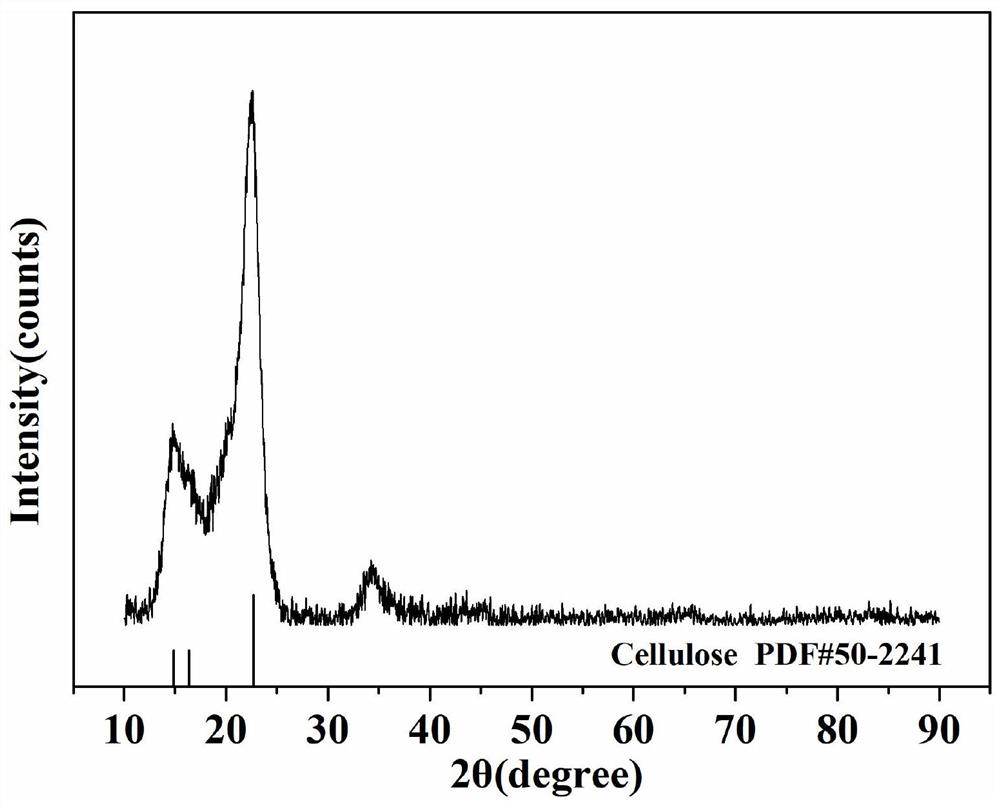
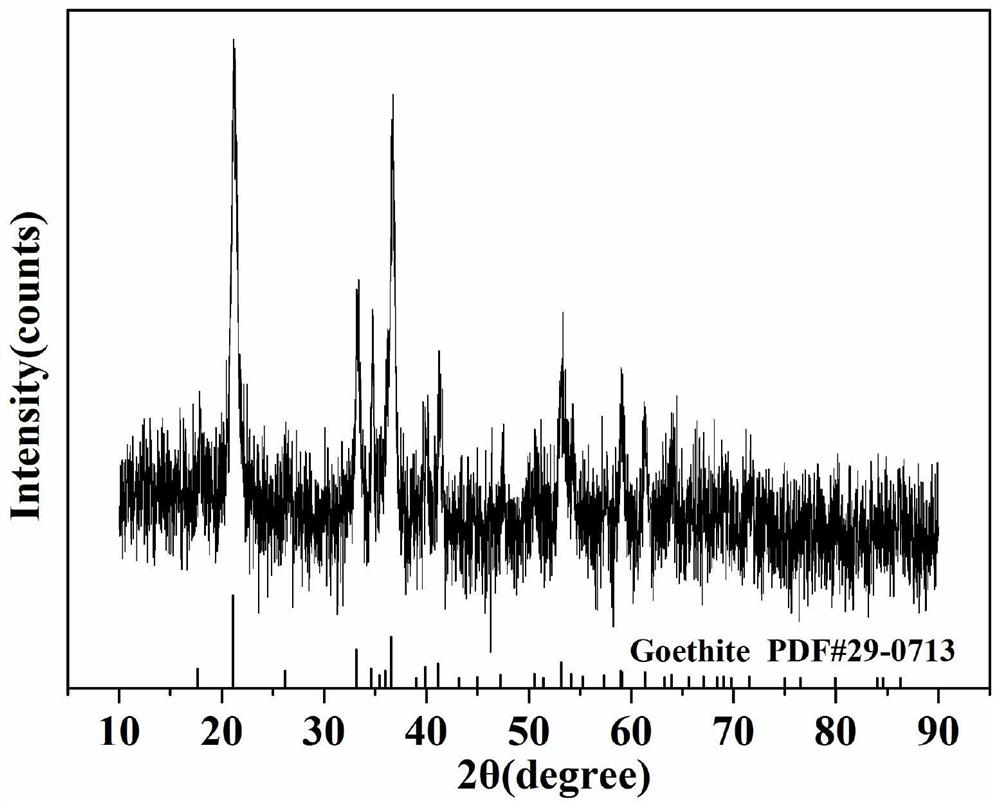
Preparation method of goethite/carboxylated cellulose nanocrystalline composite material for removing heavy metal ions
A technology of carboxylated cellulose and heavy metal ions, applied in chemical instruments and methods, other chemical processes, water/sewage treatment, etc. Stable and rigid effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0038] This embodiment provides a goethite / carboxylated cellulose nanocrystalline composite material and a preparation method thereof, comprising the following steps:
[0039] (1) At room temperature, 32.5 g of 3.2 wt % carboxylated cellulose nanocrystals were dispersed in 67.5 g of deionized water, and magnetically stirred for 20 min to form a 1 wt % carboxylated cellulose nano crystal dispersion.
[0040] (2) add 1~10g Fe(NO in the dispersion liquid that step (1) obtains 3 ) 3 9H 2 O, magnetically stirred at room temperature for 8 h to obtain Fe 3+ / carboxylated cellulose nanocrystal complex mixture.
[0041] (3) Add 5M NaOH solution to the mixed solution obtained in step (2) while stirring to adjust the pH of the mixed solution to pH>13, and continue stirring for 30 s.
[0042](4) Transfer the mixed solution obtained in the step (3) to a hydrothermal reaction kettle and seal it, place it in an oven for hydrothermal reaction at 70°C for 12 hours, and wash the product re...
Embodiment 2
[0050] (1) Disperse 32.5g of 3.2wt% carboxylated cellulose nanocrystals in 67.5g of deionized water at room temperature, and magnetically stir for 20min to form a 1wt% carboxylated cellulose nanocrystal dispersion.
[0051] (2) in the dispersion liquid that described step (1) obtains, add 1g Fe (NO 3 ) 3 9H 2 O, magnetically stirred at room temperature for 8 h to obtain Fe 3+ / carboxylated cellulose nanocrystal complex mixture.
[0052] (3) Add 5M NaOH solution to the mixed solution obtained in step (2) while stirring to adjust the pH of the mixed solution to pH>13, and continue stirring for 30 s.
[0053] (4) Transfer the mixed solution obtained in the step (3) to a hydrothermal reaction kettle and seal it, place it in an oven for hydrothermal reaction at 70°C for 12 hours, and wash the product repeatedly with deionized water after the reaction is over. properties, and then washed 2 to 3 times with absolute ethanol, placed in a vacuum drying oven at 50 ° C and dried for ...
Embodiment 3
[0060] (1) Disperse 32.5g of 3.2wt% carboxylated cellulose nanocrystals in 67.5g of deionized water at room temperature, and magnetically stir for 20min to form a 1wt% carboxylated cellulose nanocrystal dispersion.
[0061] (2) in the dispersion liquid that described step (1) obtains, add 1g Fe (NO 3 ) 3 9H 2 O, magnetically stirred at room temperature for 8 h to obtain Fe 3+ / carboxylated cellulose nanocrystal complex mixture.
[0062] (3) Add 5M NaOH solution to the mixed solution obtained in step (2) while stirring to adjust the pH of the mixed solution to pH>13, and continue stirring for 30 s.
[0063] (4) Transfer the mixed solution obtained in the step (3) to a hydrothermal reaction kettle to seal it, place it in an oven for hydrothermal reaction at 70°C for 12 hours, and wash the product repeatedly with deionized water after the reaction is over. properties, and then washed with absolute ethanol for 2 to 3 times, placed in a vacuum drying oven at 50 ° C and dried f...
PUM
| Property | Measurement | Unit |
|---|---|---|
| adsorption capacity | aaaaa | aaaaa |
| adsorption capacity | aaaaa | aaaaa |
| adsorption capacity | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com