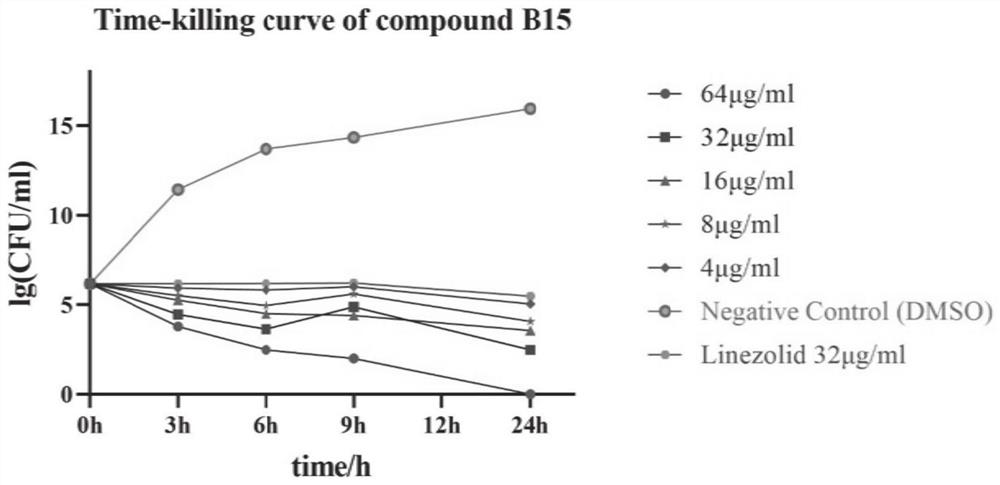
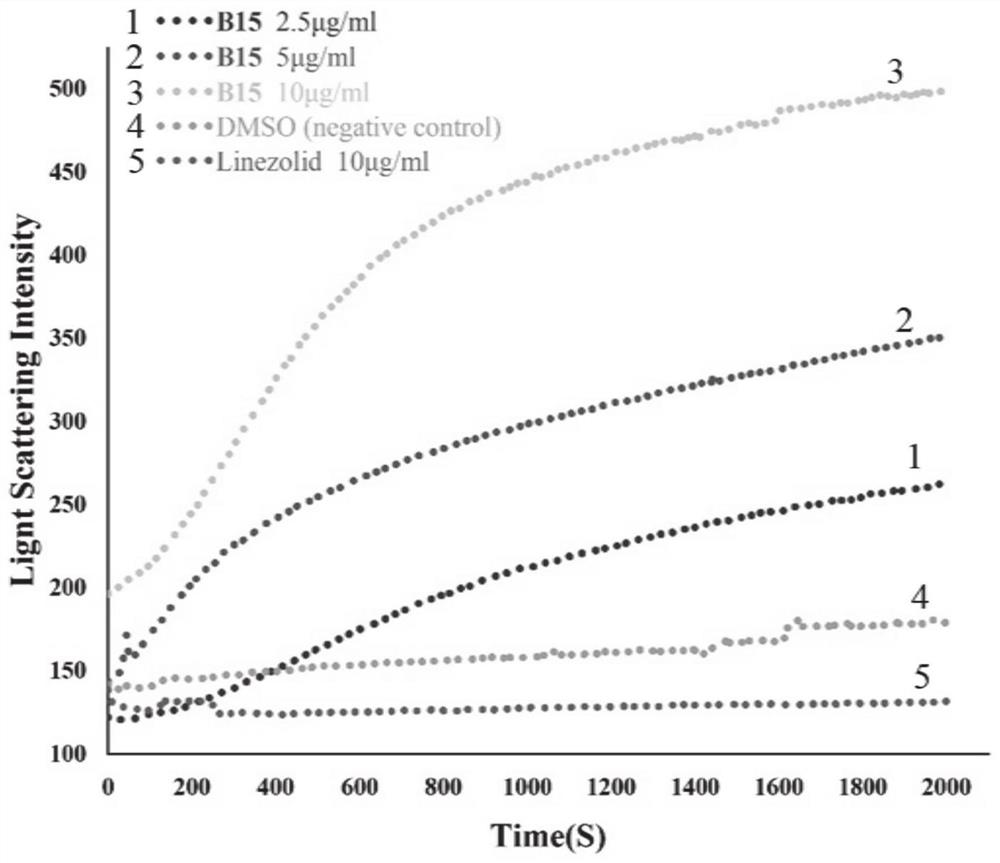
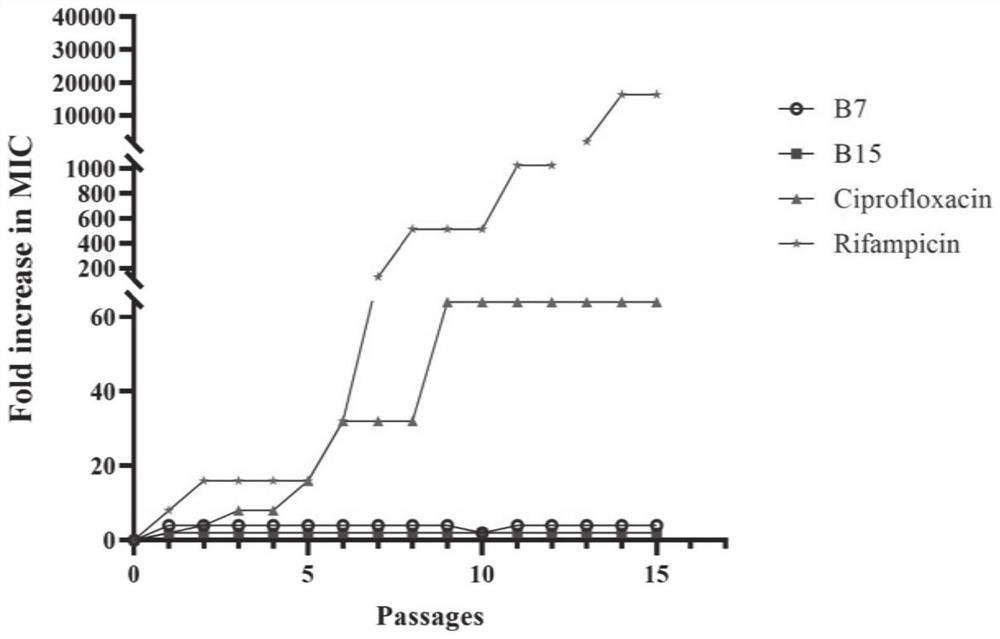
Benzoheterocycle substituted phenanthridine quaternary ammonium salt derivative as well as preparation method and application thereof
A technology of benzoheterocycle and derivatives, applied in the field of new pharmaceutical compounds, can solve problems such as insufficient druggability, and achieve the effects of good bactericidal effect, significant inhibitory effect, and significant interference effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0054] Example 1 Synthesis of 6-bromoindole intermediate 1a
[0055]
[0056] Get 6-bromoindole (1.5g, 7.65mmol) and be dissolved in DMF, N 2 Under protection, NaH (0.37g, 9.25mmol) was added at 0°C, and after stirring for 30min, methyl iodide (15.3mmol) was added, transferred to room temperature and stirred for 2h, and the reaction was complete as detected by TLC. Ethyl acetate was added to the reaction solution, and saturated NH 4 Cl solution was washed three times, saturated NaCl solution was washed three times, and dried over anhydrous magnesium sulfate. Spin dry under reduced pressure and make sand. Silica gel column chromatography (pure PE as the eluent) gave 1a as a colorless oil with a yield of 93.1%.
Embodiment 2
[0057] Example 2 Synthesis of 4-(6'-indolyl) aniline intermediate 2a
[0058]
[0059] Get the product obtained in Example 1 (1.49g, 7.12mmol), and 4-aminophenylboronic acid pinacol ester (1.72g, 7.83mmol), be dissolved in 15ml of dioxane, get potassium carbonate (4.92g, 35.6mmol ) was dissolved in 15ml of water, added to the organic phase, and finally tetrakistriphenylphosphine palladium (cat.), N 2 Protected, heated to reflux at 101°C for 12h, TLC detected that the reaction was almost complete. The reaction solution was spin-dried to dry the organic phase, added an appropriate amount of ethyl acetate for extraction, separated to remove the water phase, washed the organic phase three times with saturated NaCl solution, and dried over anhydrous magnesium sulfate. Concentrate the organic phase, silica gel column chromatography (eluent PE:EA:Et 3 N=30:1:0.1→PE:EA:Et 3 N=3:1:0.1), and 2a was obtained as a yellow oil with a yield of 65.8%.
Embodiment 3
[0060] Example 3 Synthesis of 2-bromo-3-methylbenzoyl chloride intermediate 4l
[0061]
[0062] Take 3l of 2-bromo-3-methylbenzoic acid (1.5g, 6.98mmol), dissolve it in 8ml of thionyl chloride, heat and reflux at 78°C for 2h, and TLC detects that the reaction is complete. The reaction solution was spin-dried under reduced pressure to obtain a pale yellow oil, which was directly used in the next step without purification.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com