A kind of method for preparing aldehyde based on phosphonamide phosphine ligand catalyzing internal olefin
A technology for phosphine amide and phosphine ligands is applied in the field of catalyzing internal olefins to prepare aldehydes based on phosphine amide phosphine ligands, which can solve the problems of poor aldehyde selectivity, ligand deactivation, low conversion rate and the like, and reduce production energy consumption. , increase safety, effect on water stabilization
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
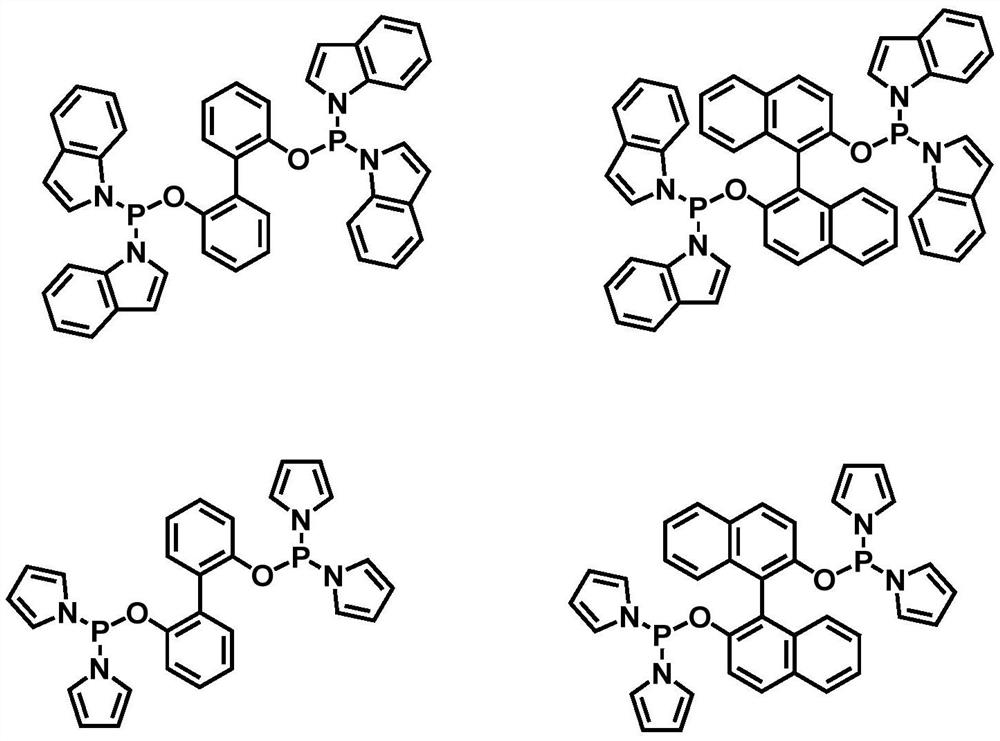
Image
Examples
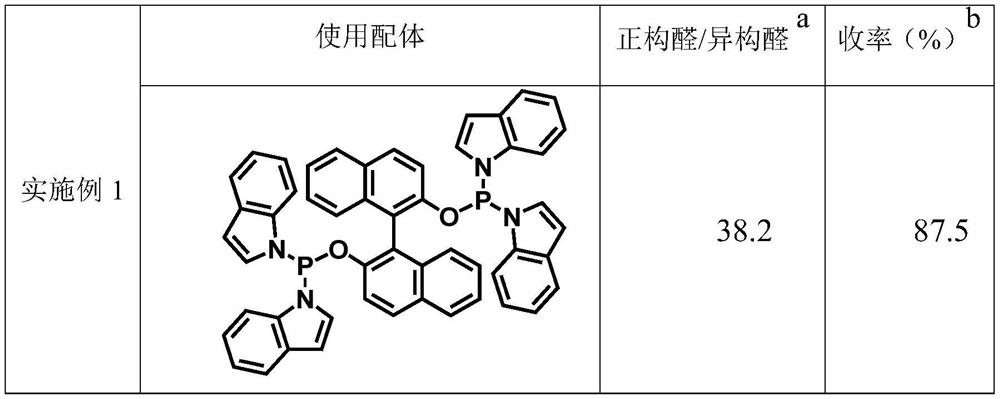
Embodiment 1
[0033] The invention provides a kind of method based on phosphine phosphine phosphine ligand and rhodium catalyst catalysis internal olefin to prepare straight chain valeraldehyde, comprises the following steps:
[0034] Combining the phosphine ligand binaphthol bisindolylphosphine with the metal rhodium precursor Rh(acac)(CO) 2 The molar ratio was 2:1 and was added to the autoclave. After adding toluene solvent, the gas was replaced with the synthesis gas mixed with hydrogen and carbon monoxide at a partial pressure ratio of 1:1. Then 5g of 2-butene was mixed with Rh (acac)(CO) 2 According to the 1064 molar ratio, a sample was taken and added to the reaction kettle. Then under the conditions of 1.0MPa (constant pressure) and 80°C, the reaction was stirred for 2h. After the reaction is completed, the product mixture is analyzed by gas chromatography, and the ratio of normal and iso-valeraldehyde is as follows:
[0035]
[0036] a molar ratio of n-aldehyde to iso-aldehyde...
Embodiment 2
[0039] The invention provides a method for catalyzing internal olefin linear valeraldehyde based on phosphine phosphine ligand, comprising the following steps:
[0040] Combining the phosphine ligand binaphthol bisindolylphosphine with the metal rhodium precursor Rh(acac)(CO) 2 The molar ratio was 5:1 and added to the autoclave, and then the xylene solvent was added, and the synthesis gas mixed with hydrogen and carbon monoxide at a partial pressure ratio of 2:1 was used to replace the gas, and then 5g of 2-butene was mixed with Rh (acac)(CO) 2A sample was taken at a 975 molar ratio and added to the reactor. Then, under the conditions of 1.0MPa (constant pressure) and 70°C, the reaction was stirred for 6h. After the reaction is completed, the product mixture is analyzed by gas chromatography, and the ratio of normal and iso-valeraldehyde is as follows:
[0041]
[0042] a is the molar ratio of n-aldehyde to iso-aldehyde;
[0043] b is the overall yield of valeraldehyde....
Embodiment 3
[0045] The present invention provides a method for preparing linear valeraldehyde based on phosphonamide phosphine ligand catalyzed internal olefin, comprising the following steps:
[0046] The phosphine ligand binaphthol bis-indolyl phosphine and the metal rhodium precursor Rh(COD)(acac) were added to the autoclave at a molar ratio of 10:1, and after adding trimethylbenzene solvent, hydrogen and carbon monoxide were used to press The synthesis gas mixed with a partial pressure ratio of 0.8:1 was subjected to the replacement gas operation, and then 5 g of 2-butene and Rh(COD)(acac) were sampled at a molar ratio of 2000 and added to the reaction kettle. Then under the conditions of 1.5MPa (constant pressure) and 80°C, the reaction was stirred for 6h. After the reaction is completed, the product mixture is analyzed by gas chromatography, and the ratio of normal and iso-valeraldehyde is as follows:
[0047]
[0048]
[0049] a is the molar ratio of n-aldehyde to iso-aldehy...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com