Synthesis method of N-methylisopropylamine
A technique for synthesizing methylisopropylamine, which is applied in the preparation of pharmaceutical intermediates and the field of pesticides, can solve the problems of high price, harsh reaction conditions, and high corrosion of equipment, and achieve control of conversion rate and selectivity, simple reaction steps, The effect of low output of three wastes
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
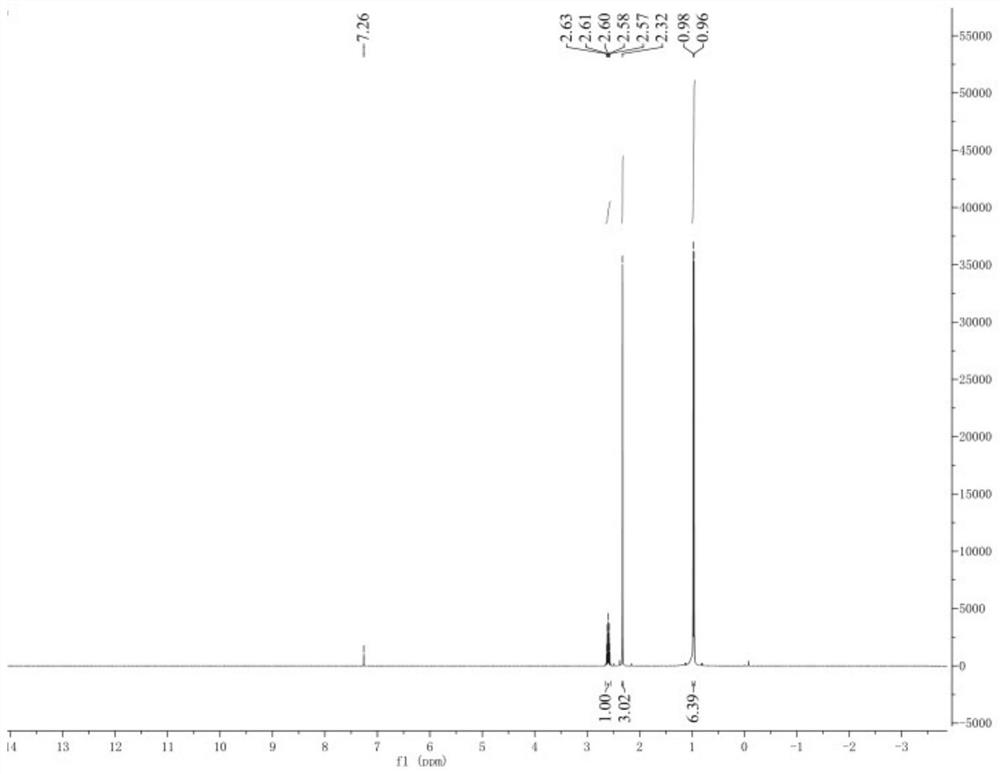
[0027] Add 100 g of dimethyl carbonate, 15 g (0.254 mol) of isopropylamine, and 0.3 g of alkaline kaolin as a catalyst into a 250 ml reaction flask, stir and heat up to reflux under normal pressure, and the reflux time is 16 hours. The reaction process was followed by GC until the disappearance of the raw materials, and the reaction was considered to be over. Afterwards, the material was cooled to room temperature and filtered to remove the catalyst. The filtrate passed through a 20-30 cm rectification column, rectified at normal pressure, and collected the main fraction at 42-45 ° C to obtain 17.95 g of N-methylisopropylamine. The appearance of the material was colorless and transparent, and the GC purity was 99.21 %, the yield after rectification is 96.69%, and the by-product N,N-dimethylisopropylamine is 0.43%.
Embodiment 2
[0029] Add 100 g of dimethyl carbonate, 15 g (0.254 mol) of isopropylamine, and 0.75 g of catalyst alkaline montmorillonite into a 250 ml reaction flask, stir and heat up to reflux under normal pressure, and the reflux time is 16 hours. The reaction process was followed by GC until the disappearance of the raw materials, and the reaction was considered to be over. Afterwards, the material was cooled to room temperature and filtered to remove the catalyst. The filtrate passed through a 20-30cm rectification column, rectified at normal pressure, and collected the main fraction at 42-45°C to obtain 18.10g of N-methylisopropylamine. The appearance of the material was colorless and transparent, and the GC purity was 99.10 %, the yield after rectification is 97.51%, and the by-product N,N-dimethylisopropylamine is 0.56%.
Embodiment 3
[0031] Add 100 g of dimethyl carbonate, 15 g (0.254 mol) of isopropylamine, and 0.75 g of alkaline kaolin into a 250 ml reaction flask, stir and heat up to reflux under normal pressure, and the reflux time is 18 hours. The reaction process was followed by GC until the disappearance of the raw materials, and the reaction was considered to be over. Afterwards, the material was cooled to room temperature and filtered to remove the catalyst. The filtrate was rectified through a 20-30 cm rectification column at atmospheric pressure, and the main fraction at 42-45 ° C was collected to obtain 18.27 g of N-methylisopropylamine. The appearance of the material was colorless and transparent, and the GC purity was 98.63 %, the yield after rectification is 98.43%, and the by-product N,N-dimethylisopropylamine is 1.04%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com