Programmable intelligent controlled-release capsule as well as application and preparation method thereof
An intelligent controlled release and capsule technology, which is applied in the directions of non-active ingredient medical preparations, capsule delivery, pharmaceutical formulations, etc., can solve the problems of delayed release, immediate release, and poor shape of drug printing, and achieves reduction of processing procedures, shortening Processing cycle, the effect of simple process
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
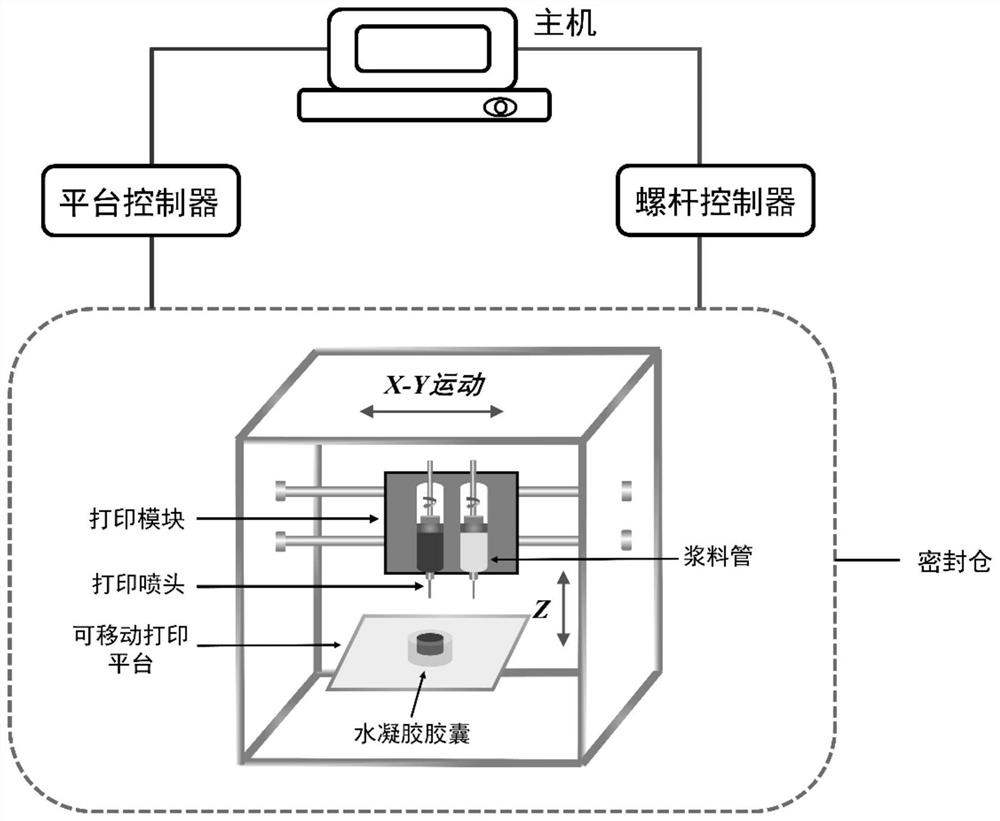
[0040] The preparation method of the programmable intelligent controlled-release capsule provided by the present invention comprises the following steps:
[0041] Step 1, the shell material of the capsule and the drug of the pretreated inner core are respectively loaded into two slurry tubes of the 3D printing system as slurry;
[0042] Step 2, using modeling software to build a three-dimensional model of the capsule, and then transfer it to the 3D printing system after conversion by slicing software;
[0043] Step 3. Set the parameters and start the 3D printing system to accurately form the first layer of the capsule structure slice on the substrate, and then move the substrate upwards, and the moving height is the same as the layer thickness of the first layer. Print the second layer on the basis of the above, and repeat until the entire capsule structure is formed.
[0044] Step 4: curing the molded capsule structure to obtain a programmable and controlled-release intellig...
Embodiment 1
[0052] Embodiment 1: the preparation shell thickness is the capsule of 2mm
[0053] 1. Material preparation:
[0054] (1) Preparation of capsule shell material: add the above materials into 10ml according to the molar ratio of NIPAM:N,N-methylenebisacrylamide:α-ketoglutaric acid:NaOH=5000:1:5:125 Mix in pure water evenly, vacuum degass under stirring, then add 1.5% Carbomer 940 to the ink, after fully stirring, the shell printing ink for 3D printing is obtained;
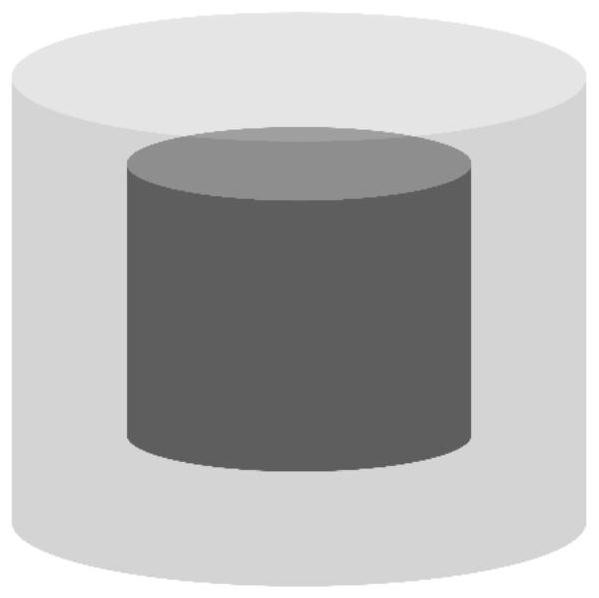
[0055] (2) Pretreatment of the inner core drug: add the above materials into 10ml of pure water in the ratio of Brilliant Blue: NaOH=16:125 by molar ratio, mix evenly, vacuum degas under stirring, and then add 1.5% Carbomer to the ink 940, after fully stirring, obtain the medicinal printing ink that is used for 3D printing, here, bright blue is used as medicine model ( figure 1 mid-dark part).
[0056] 2. Preparation process
[0057] (1) First, use Solidworks software to establish a three-dimensional solid model ...
Embodiment 2
[0064] Embodiment 2: the preparation shell thickness is the capsule of 4mm
[0065] The steps of this example differ from Example 1 in that the thickness of the outer shell of the prepared capsule is 4 mm, and the drug size of the inner core is the same as that of Example 1.
PUM
| Property | Measurement | Unit |
|---|---|---|
| length | aaaaa | aaaaa |
| thickness | aaaaa | aaaaa |
| thickness | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com