Methods of treating lung cancer with a pd-1 axis binding antagonist, an antimetabolite, and a platinum agent
An anti-metabolite, PD-L1 technology, applied in chemical instruments and methods, anti-animal/human immunoglobulin, antibody medical components, etc., can solve the problems of low survival rate of advanced NSCLC disease
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
example
[0423] The present disclosure will be more fully understood with reference to the following examples. However, they should not be construed as limiting the scope of the invention. It should be understood that the examples and embodiments described herein are for illustrative purposes only, and various modifications or changes in view thereof will be suggested to those skilled in the art, and will be included within the spirit and scope of the application and the scope of the present invention. within the scope of the appended claims.
example 1
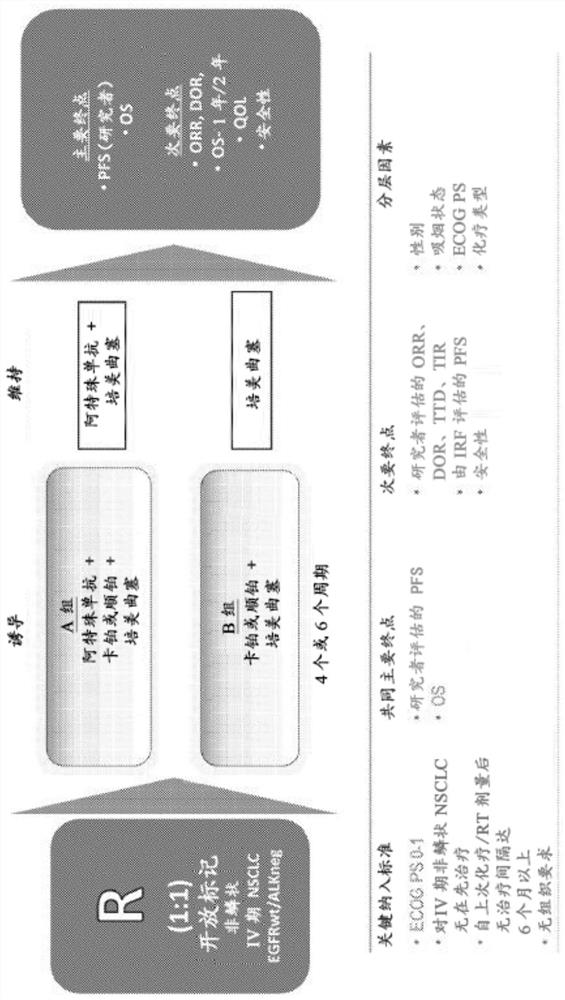
[0424] Example 1: Atezolizumab in combination with carboplatin + pemetrexed or cisplatin + pemetrexed in chemotherapy-naive patients with stage IV non-squamous non-small cell lung cancer+ (NSCLC)+ Phase III open-label randomized study comparing carboplatin-pemetrexed or cisplatin-pemetrexed
[0425]This study aimed to evaluate atezolizumab plus carboplatin+pemetrexed+or cisplatin+pemetrexed versus carboplatin+pemetrexed or cisplatin-pemetrexed for chemotherapy-naïve Comparison of efficacy, safety and pharmacokinetics in patients with stage IV non-squamous non-small cell lung cancer (NSCLC). The subsection outlines the specific goals and corresponding endpoints of the study.
[0426] Research objectives
[0427] The co-primary efficacy objectives of the study are as follows:
[0428] · Death from any cause according to RECIST v1.1 (see for example Eisenhauer et al., (2009) "Newresponsevaluation criteria in solid tumors: Revised RECIST guideline (Version 1.1)." EurJ Cancer.45...
example 2
[0628] Example 2: Efficacy of atezolizumab in combination with carboplatin-pemetrexed or cisplatin-pemetrexed as first-line therapy in key subgroups of patients with stage +IV+ non-squamous non-small cell lung cancer (NSCLC)
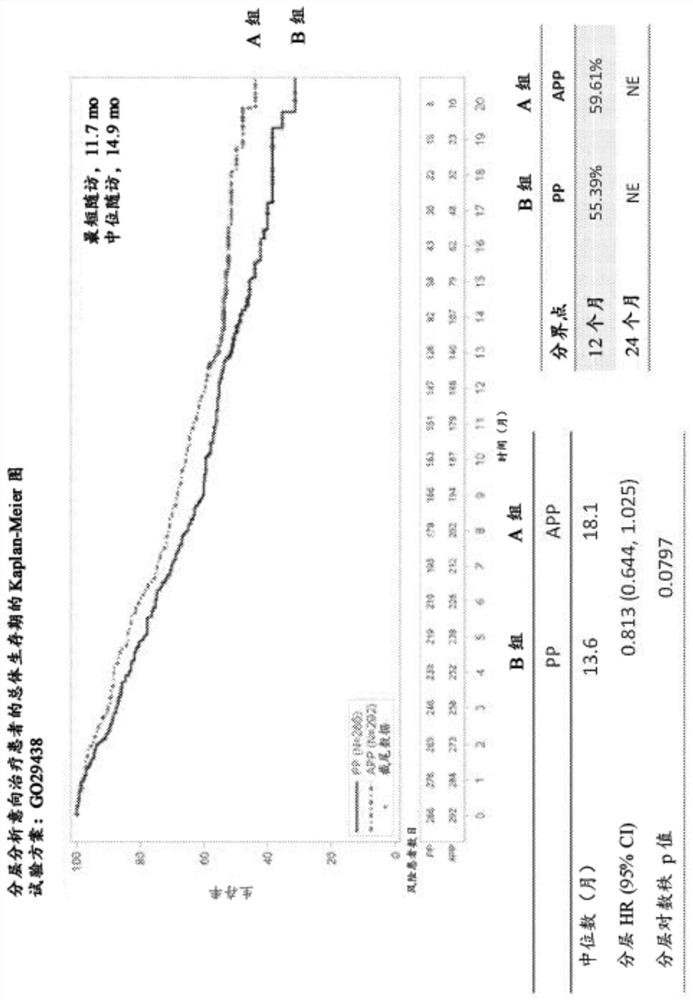
[0629] Based on the results described in Example 1, exploratory efficacy analyzes were performed on PFS and interim OS in clinically relevant patient subgroups (eg, race, age, smoking history, and baseline liver metastases).
[0630] 578 patients were enrolled. The median follow-up time was 14.8 months. Baseline characteristics were generally balanced between treatment groups. See Table 12 and Figure 5B , which are PFS and interim OS data in key subgroups.
[0631] Table 12: PFS and interim OS data in key subgroups
[0632]
[0633]
[0634] The addition of atezolizumab to carboplatin or cisplatin plus pemetrexed improved PFS and OS values in most key clinical subgroups. The survival benefit appeared to be more pronounced in Asian patients,...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com