Organic compound as well as electronic element and device using same
An organic compound and fusion technology, applied in the field of organic electroluminescent devices, can solve the problems of organic layer materials that have not yet been developed
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment approach
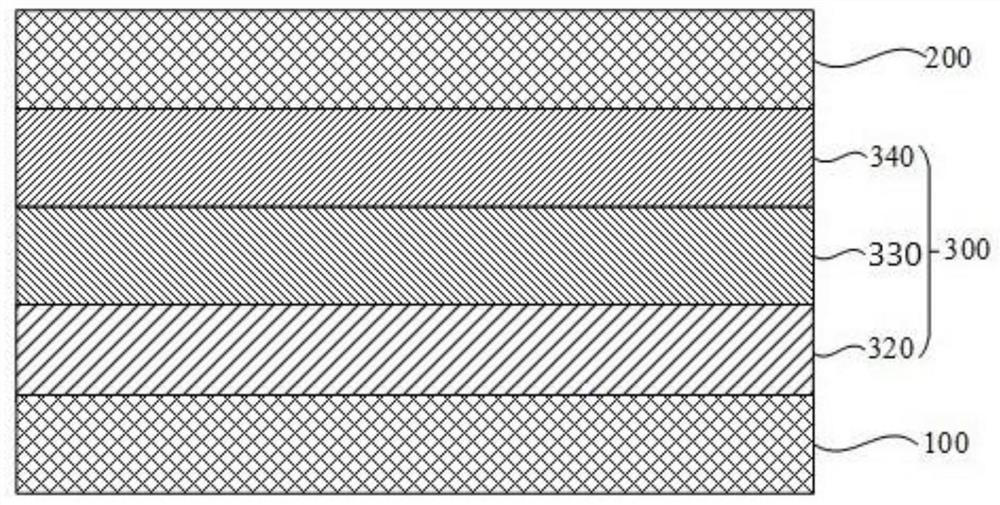
[0178] According to an exemplary embodiment, as image 3 As shown, the functional layer 300 includes an organic light emitting layer 330, and the organic light emitting layer 330 includes the organic compound of the present application. Wherein, the organic light-emitting layer 330 can be composed of the organic compound provided in this application, or can be composed of the organic compound provided in this application and other materials.
[0179] Optionally, the organic light emitting layer 330 may also include inorganic doping materials to improve the light emitting performance of the organic light emitting layer 330 .
[0180] According to a specific embodiment, such as image 3 As shown, the photoelectric conversion device may include an anode 100 , a hole transport layer 320 , an organic light emitting layer 330 , an electron transport layer 340 and a cathode 200 which are sequentially stacked.
[0181]Optionally, the photoelectric conversion device may be a solar ce...
preparation example 1
[0190] Preparation Example 1 Preparation of Compound 1
[0191] 1) Synthesis of Intermediate 1-1
[0192]
[0193] Add 1-adamantanol (50.0g, 328.4mmol), bromobenzene (51.6g, 328.4mmol), dichloromethane (DCM, 500mL) into a round-bottomed flask, and cool down to -5~0°C under nitrogen protection. Add trifluoromethanesulfonic acid (73.9g, 492.6mmol) dropwise at -5~0°C, keep stirring for 3h; add deionized water (300mL) to the reaction solution and wash until pH=7, add dichloromethane (100mL) to carry out Extract, combine the organic phases, dry using anhydrous magnesium sulfate, filter, and remove the solvent under reduced pressure; the resulting crude product is purified by silica gel column chromatography using n-heptane as the mobile phase to obtain white solid intermediate 1-1 (52.6g, 55% ).
[0194] Intermediates 1-2 to 1-3 were prepared in the same manner as Intermediate 1-1, except that starting material A in Table 1 was used instead of bromobenzene, the starting materi...
preparation example 2-6
[0204] The following compound was prepared by the same synthetic method as Preparation Example 1, except that the raw material B in Table 2 was used instead of 2-bromo-4,6-diphenyl-[1,3,5]tri Oxyzine, using Intermediate C instead of Intermediate 1-1 in Preparation Example 1. The main raw materials used, the synthesized compounds and their yields and mass spectrometry results are shown in Table 2.
[0205] Table 2
[0206]
[0207]
[0208] NMR data of compound 11
[0209] 1 H NMR (400MHZ, dichloromethare-D 2 ):δ9.25(s,1H),δ8.95(s,1H),δ8.53-8.51(m,2H),δ8.30(d,1H),δ8.11(d,1H),δ7 .98(d,1H),δ7.95(s,1H),δ7.72(d,1H),δ7.64-7.50(m,13H),δ7.45-7.40(m,4H),δ7. 35-7.28(m,6H),δ2.20(s,3H),δ1.96(s,6H),δ1.84-1.74(m,6H).
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com