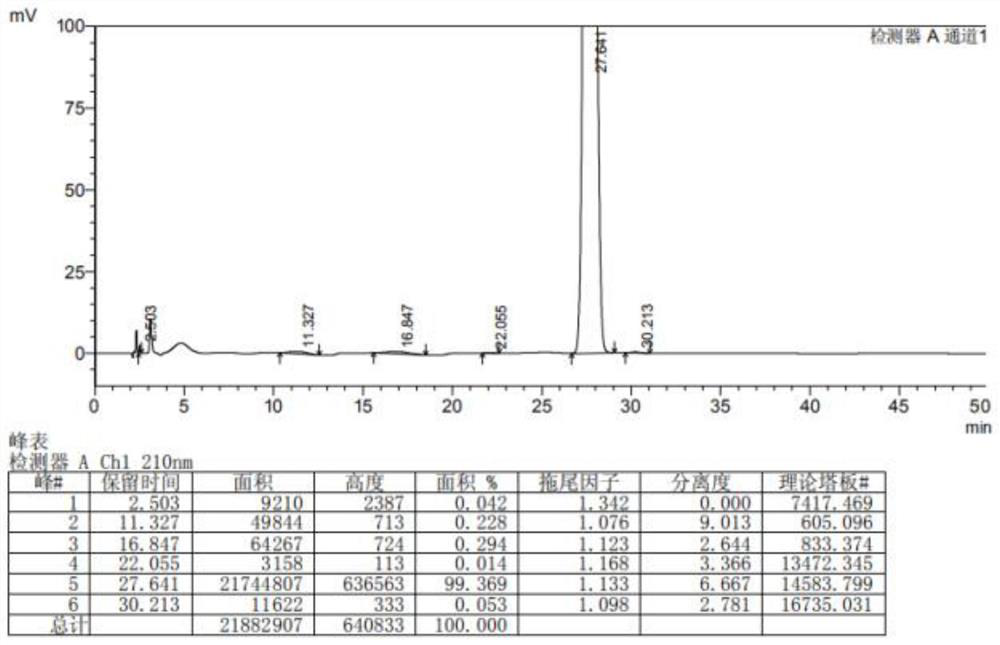
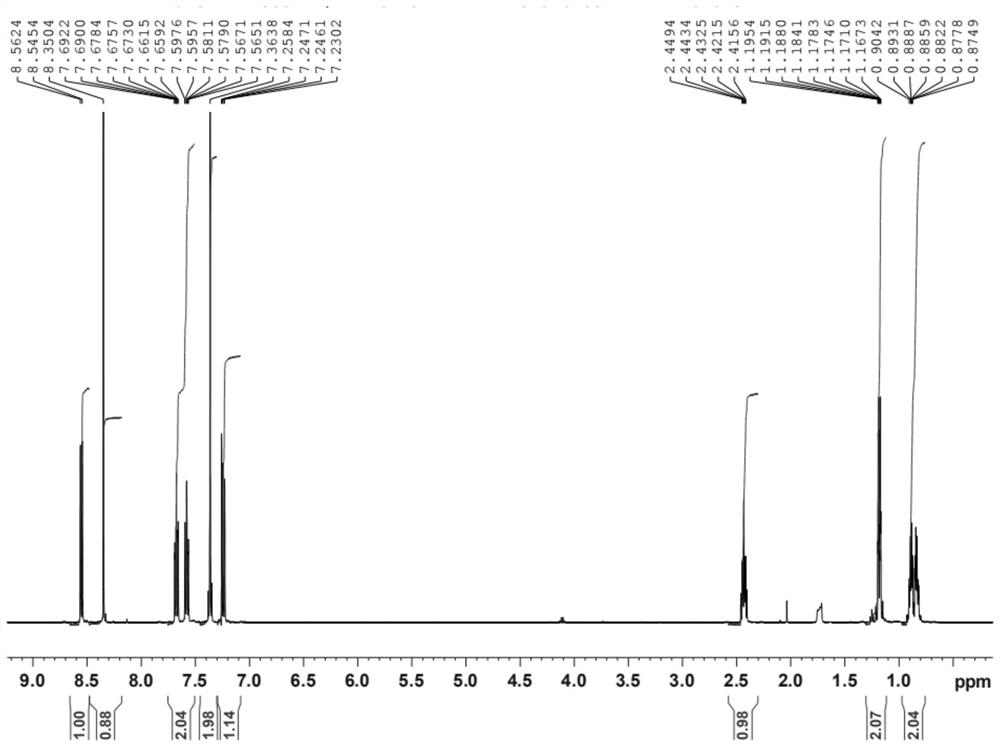
Preparation method of lesinurad oxidation impurity
A technology for oxidizing impurities and compounds, which is applied in the field of preparation of oxidized impurities in Recinard, and can solve the problems of oxidized impurities and other problems
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
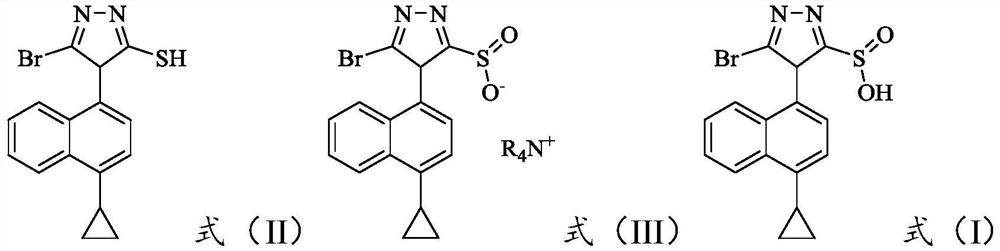
[0034] The invention provides a method for preparing the oxidized impurities of Lecinard, comprising: S1) oxidizing the compound represented by the formula (II) under alkaline conditions, then adjusting the pH value of the reaction solution to acidity, and then reacting with the quaternary ammonium salt reaction to obtain a compound shown in formula (III); S2) dissolving the compound shown in formula (III) in a mixed solvent of water and the first organic solvent, adjusting the pH value to acidity, and then separating the organic phase Solvent is removed to obtain the oxidized impurity of Leycinard shown in formula (I);
[0035]
[0036] Wherein, R is a C1-C10 alkyl group, preferably a C2-C8 alkyl group, more preferably a C3-C6 alkyl group, and still more preferably a C4-C5 alkyl group.
[0037] Taking R as a butyl group as an example, the synthetic route is:
[0038]
[0039] The compound represented by formula (II) is oxidized under basic conditions; the reaction is p...
Embodiment 1
[0053] Step 1: Add 100ml of purified water and sodium hydroxide (1.15g, 0.0289mol) to a 1L three-necked flask, stir to dissolve, and then add 5-bromo-4-(4-cyclopropylnaphthalene- 1-yl)-4H-1,2,4-triazole-3-thiol (10.00g, 0.0289mol), heated to reflux, then added potassium permanganate (9.13g, 0.0578mol), and refluxed to complete , cool to room temperature, filter, collect the filtrate, adjust the filtrate to pH 5-6 with acetic acid, add tetrabutylammonium acetate (13g, 0.0434mol) to the filtrate, stir until the reaction is complete, then use 200ml, 100ml dichloro Extract the reaction solution with methane, combine the organic phases, dry over anhydrous sodium sulfate, filter and concentrate to dryness, add 50ml of methyl isobutyl ketone and stir to dissolve, slowly cool down to 7°C for crystallization for 3 hours, filter, and vacuum-dry at 50°C to obtain a light yellow solid .
[0054] Add the light yellow solid obtained in step 1 to a 500ml three-neck flask, heat 200ml of ethy...
Embodiment 2
[0057] Step 1: Add 500ml of purified water and sodium hydroxide (1.15g, 0.0289mol) to a 1L three-necked flask, stir to dissolve, and then add 5-bromo-4-(4-cyclopropylnaphthalene- 1-yl)-4H-1,2,4-triazole-3-thiol (10.00g, 0.0289mol), heated to reflux, then added 30% hydrogen peroxide (11.6ml, 0.1156mol), and refluxed to complete, cool down to room temperature, filter, collect the filtrate, adjust the filtrate to pH 5-6 with acetic acid, add tetrabutylammonium acetate (13g, 0.0434mol) to the filtrate, stir until the reaction is complete, then use 200ml, 100ml Extract the reaction solution with methyl chloride, combine the organic phases, dry over anhydrous sodium sulfate, filter and concentrate to dryness, add 50ml of methyl isobutyl ketone and stir to dissolve, slowly cool down to 6°C for crystallization for 3 hours, filter, and vacuum-dry at 50°C to obtain light yellow solid.
[0058] Add the light yellow solid obtained in step 1 to a 500ml three-necked flask, heat 230ml of is...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Wavelength | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com