Method for preparing quinazolone and derivatives thereof by using chitosan-loaded copper catalyst
A technology of copper catalyst and chitosan, which is applied in the field of compound synthesis, can solve problems such as long reaction time, large environmental pollution, and residues
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
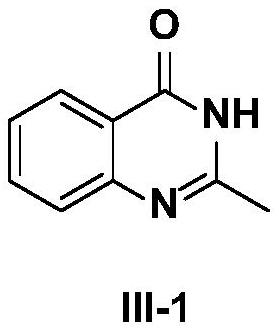
[0032] The preparation method of compound III-1, its steps are:
[0033]
[0034] A. Add a magnetic stirrer in the reaction tube, seal it and connect it to a vacuum device; evacuate for 5 minutes and then continue to feed argon for 1 min. Repeat the above operation 3 times to completely remove the oxygen in the reaction tube; after continuously feeding argon Add 5 mg of chitosan-supported cuprous iodide (CS@CuI) (0.00001mol) and starting material I-1 to the reaction tube in sequence: (124.0mg, 0.5mmol), II-1: (79.1mg, 0.75mmol) and sodium carbonate (132.5mg, 1.25mmol), dissolved in 3mL mixed solvent (isopropanol: water = 9:1), stirred at 90°C for 12h;
[0035] B. After the reaction is finished, filter the entire reaction system and wash with 20 mL of ethyl acetate. Chitosan-supported cuprous iodide (CS@CuI) was recovered by filtration, and the filtrate was evaporated and concentrated, and the residue was separated and purified by column chromatography with different pro...
Embodiment 2
[0040] The preparation method of compound III-2, its steps are:
[0041]
[0042] A. Add a magnetic stirrer in the reaction tube, seal it and connect it to a vacuum device; evacuate for 5 minutes and then continue to feed argon for 1 min, repeat the above operation 3 times and completely remove the oxygen in the reaction tube; after continuously feeding argon Add 5 mg of chitosan-supported cuprous iodide (CS@CuI) (0.00001mol) and starting material I-1 to the reaction tube in sequence: (124.0mg, 0.5mmol), II-2: (89.7mg, 0.75mmol) and sodium carbonate (132.5mg, 1.25mmol), dissolved in 3mL mixed solvent (isopropanol:water=9:1)), stirred at 90°C for 12h;
[0043] B. After the reaction is finished, filter the entire reaction system and wash with 20 mL of ethyl acetate. Chitosan-supported cuprous iodide (CS@CuI) was recovered by filtration, and the filtrate was evaporated and concentrated. The residue was separated and purified by column chromatography with different proporti...
Embodiment 3
[0047] The preparation method of compound III-3, its steps are:
[0048]
[0049] A. Add a magnetic stirrer in the reaction tube, seal it and connect it to a vacuum device; evacuate for 5 minutes and then continue to feed argon for 1 min, repeat the above operation 3 times and completely remove the oxygen in the reaction tube; after continuously feeding argon Add 5 mg of chitosan-supported cuprous iodide (CS@CuI) (0.00001mol) and starting material I-1 to the reaction tube in sequence: (124.0mg, 0.5mmol), II-3: (101.3mg, 0.75mmol) and sodium carbonate (132.5mg, 1.25mmol), dissolved in 3mL mixed solvent (isopropanol: water = 9:1), stirred at 90°C for 12h;
[0050] B. After the reaction is finished, filter the entire reaction system and wash with 20 mL of ethyl acetate. Chitosan-immobilized cuprous iodide (CS@CuI) was recovered by filtration, and the filtrate was evaporated and concentrated. The residue was separated and purified by column chromatography with different pro...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com