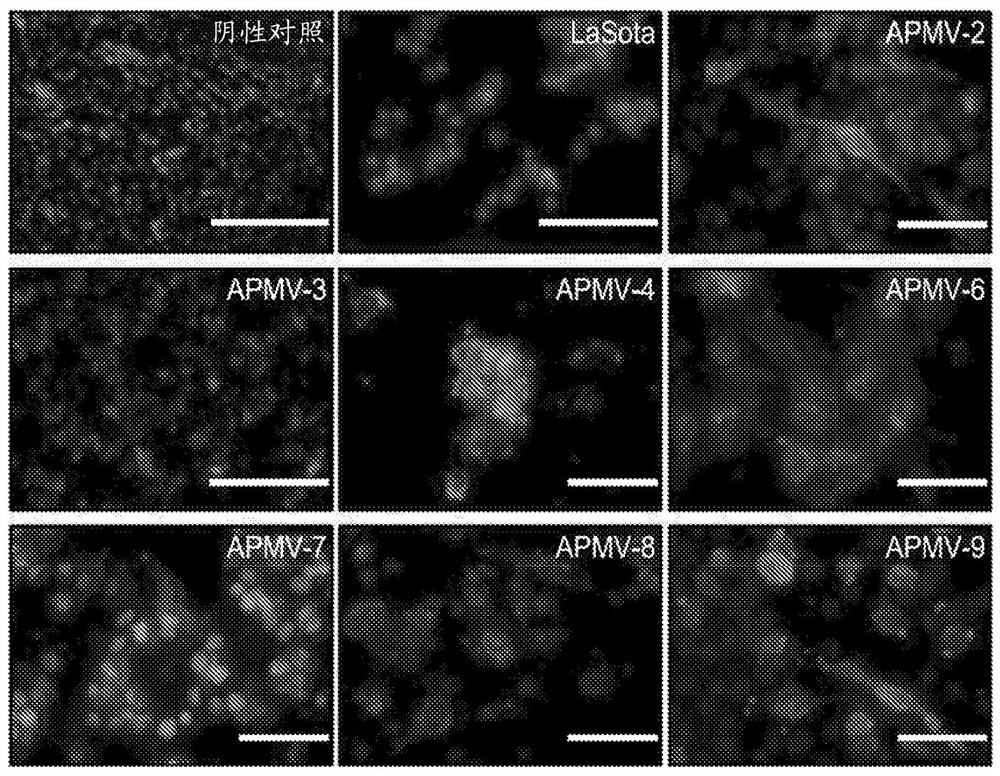
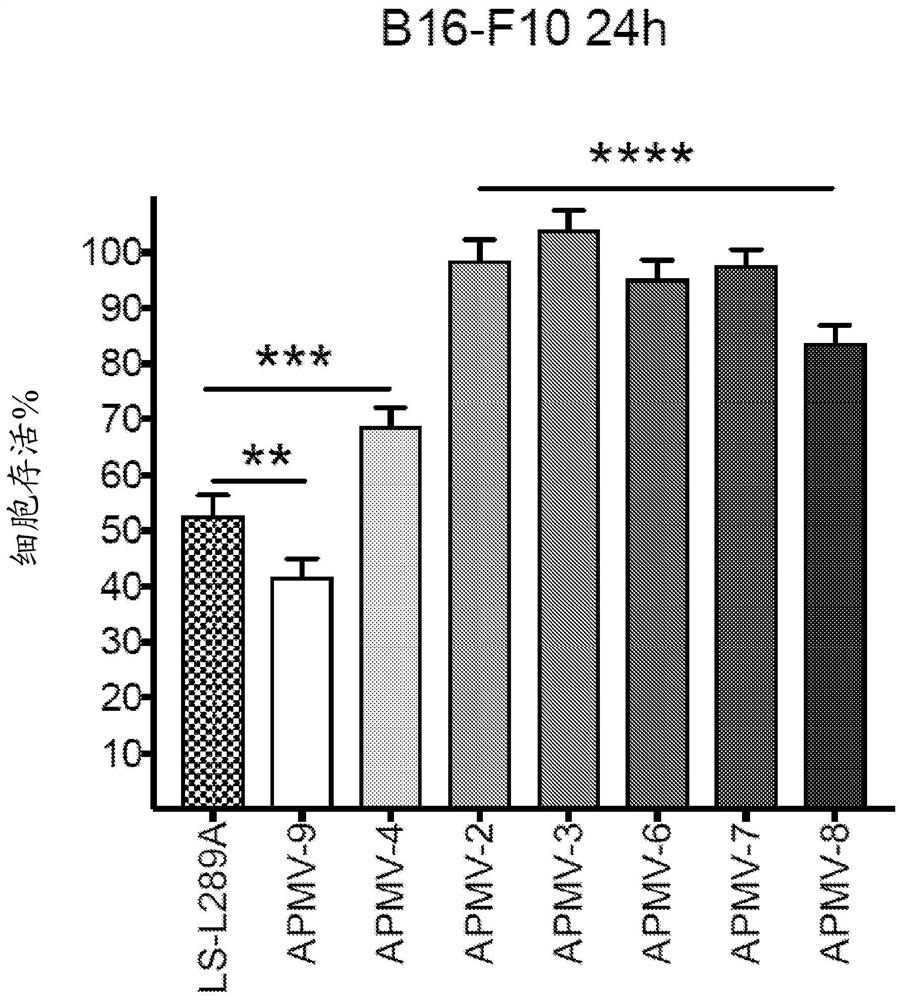
Apmv and uses thereof for treatment of cancer
一种APMV-4、癌症的技术,应用在优先权益领域
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment approach
[0506] The following exemplary implementations are provided herein:
[0507] 1. A method for the treatment of cancer, comprising administering to a human subject in need thereof a naturally occurring avian paramyxovirus serotype 4 (APMV-4), wherein the APMV-4 is present within 1 day of the Gallus gallus species - Intracerebral inoculation pathogenicity index less than 0.7 in large chicks.
[0508] 2. A method of treating cancer, comprising administering recombinant APMV-4 to a human subject in need thereof, wherein said recombinant APMV-4 is inoculated in the brain in 1 day-old chicks of Gallus gallus species The pathogenicity index was less than 0.7.
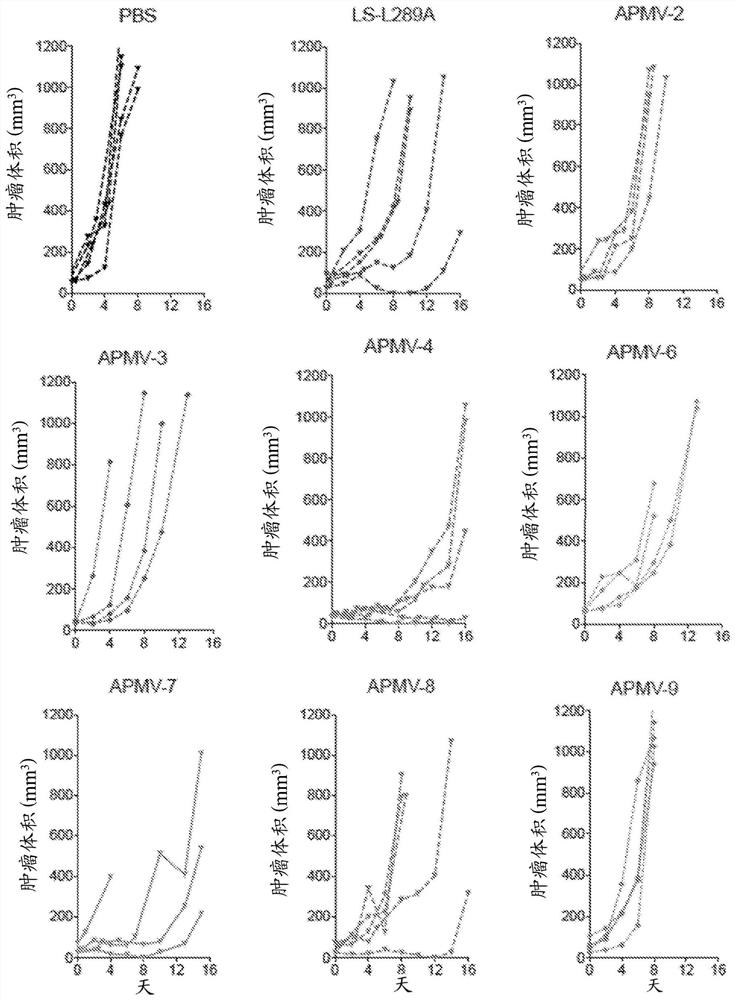
[0509] 3. The method of embodiment 1 or 2, wherein administration of APMV-4 reduces tumor growth and survival in a B16-F10 syngeneic murine melanoma model administered with phosphate buffered saline (PBS) demonstrated tumor growth and improved survival in a B16-F10 syngeneic murine melanoma model.
[0510] 4. The method of e...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com