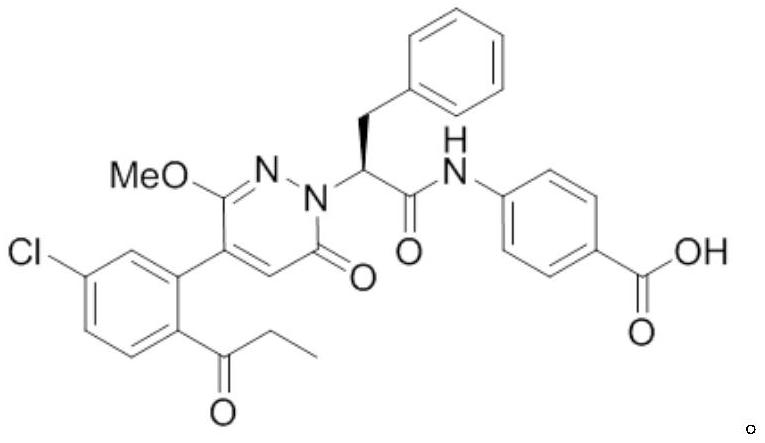
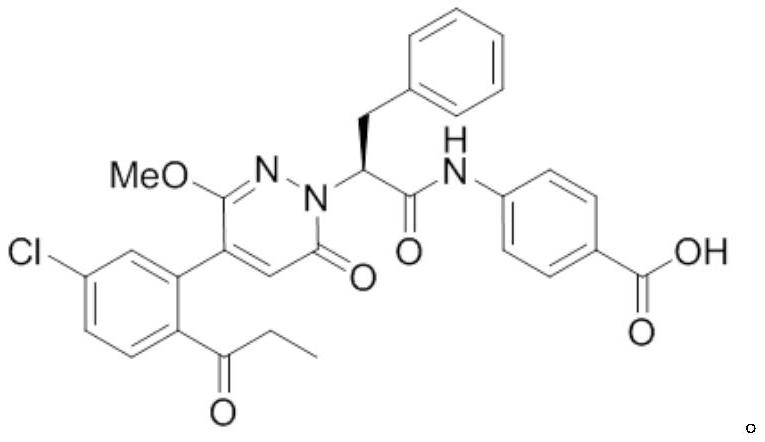
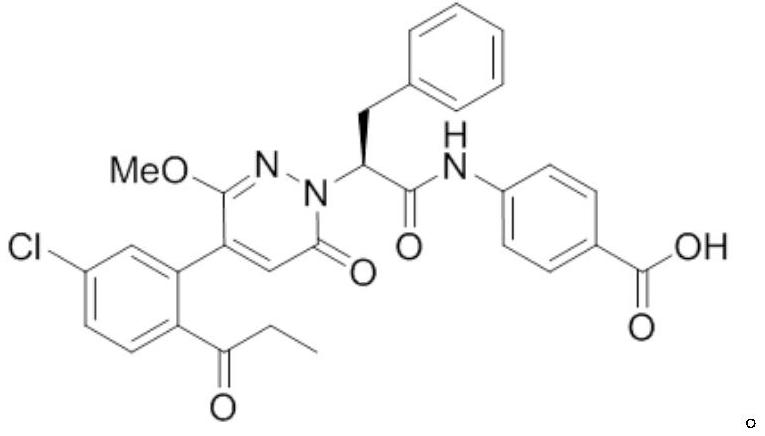
Medicine purpose of FXIa inhibitor compound or salt thereof
A technology of compounds and inhibitors, applied in the field of preparation of drugs for the prevention and/or treatment of arterial and venous thrombosis, capable of solving problems such as negligible effects
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0040] Compound of Example 1
[0041] Concrete synthetic route is as follows:
[0042] Step A: Synthesis of 1-(2-bromo-4-chlorophenyl)propan-1-one
[0043]
[0044] 2-Bromo-4-chloro-1-iodobenzene (2.00 g, 6.30 mmol) was dissolved in 2 mL of THF by a known method (Angewandte Chemie, International Edition, 2010, 49(46), 8729-8732) , cooled to -20°C, a solution of isopropylmagnesium chloride in n-hexane (2M concentration) (4.1 ml, 8.2 mmol) was added dropwise, and stirred at this temperature for 1 hour.
[0045] Propionyl chloride (716 μl, 8.20 mmol), lithium chloride (23 mg, 378 μmol), cuprous chloride (19 mg, 189 μmol), and aluminum trichloride (25 mg, 189 μmol) Add 2 ml of tetrahydrofuran, stir evenly at room temperature, cool in an ice-water bath, slowly add the above reaction solution that has been reacted for one hour in advance to the above mixture, and react at room temperature for two hours after addition. Add 40 milliliters of saturated ammonium chloride solution ...
Embodiment 2
[0084] : Absorption light method detects the biological activity of the compounds of the present invention inhibiting human blood coagulation factor XIa
[0085] 1. Experimental materials
[0086] Enzyme: Human Factor XIa (ENZYME RESEARCH, Cat. No. HFXIa 1111a)
[0087] Substrate: S-2366 TM : (CHROMOGENIX, Cat. No. 82109039)
[0088] Buffer: 145mM NaCl, 5mM KCl, 1mg / mL PEG 8000, 30mM HEPES, pH7.4
[0089] 2. Experimental steps
[0090] 10mM test compound dissolved in 100% DMSO was diluted with 100% DMSO to 1000, 200, 40, 8, 1.6, 0.32, 0.064, 0.0128, 0.00256, 0.00128μM; 98μL (77.7ng / mL) of FXIa enzyme solution, the blank wells were replaced by 98 μL of buffer, and then 2 μL of compounds of different concentrations were added, the blank and control wells were replaced by DMSO, mixed with a shaker, and incubated at 37 ° C for 20 min.
[0091] Finally, 100 μL of 800 μM substrate was added to each well, and its absorbance was measured at 405 nm.
[0092] 3. Data processing ...
Embodiment 3
[0097] Example 3: Determination of the compound of the present invention on the anticoagulant effect of human plasma in vitro
[0098] 1. Experimental materials
[0099] Plasma: Human blood was collected in vacuum blood collection tubes containing 3.2% sodium citrate (volume ratio 1:9), centrifuged at room temperature at 3000rpm for 10min, collected plasma, aliquoted into EP tubes, and stored at -80°C.
[0100] Reagents: APTT assay kit (activated partial thromboplastin time assay kit, mindray), calcium chloride solution.
[0101] Instrument: coagulation instrument (mindray, C2000-A)
[0102] 2. Experimental method
[0103] After thawing the aliquoted frozen human plasma at room temperature, mix well. Dilute 10mM test compound dissolved in 100% DMSO with 100% DMSO to 1500, 750, 375, 187.5, 93.75, 46.88, 23.44, 11.72μM; add 98μL of human plasma to a 1.5mL EP tube, and then add 2μL of different concentrations Add 2 μL of 100% DMSO to the blank group, incubate in a water bath ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com