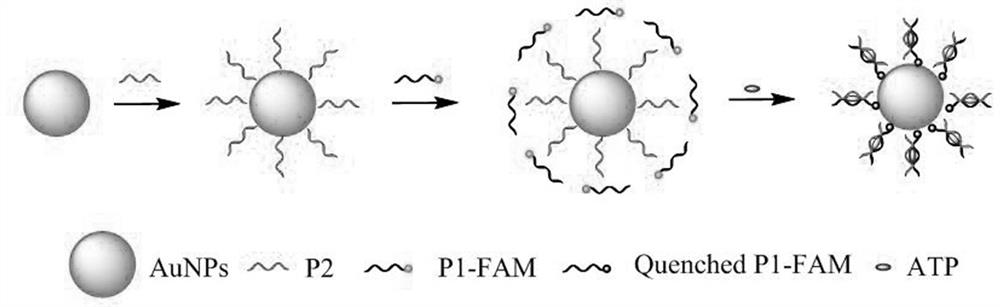
Split aptamer sensor based on nanogold as well as preparation method and application of sensor
An aptamer sensor and nano-gold technology, applied in the field of sensors, can solve the problems of high detection limit, high operation difficulty, weakened affinity, etc., and achieve the effects of low detection limit, simple operation and good specificity.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0041]The preparation method based on nano-gold split body sensor, the steps are as follows:
[0042]5 μmol / L modified sulfhydryl groups were activated at 10 mmol / L, pH = 7 TRIS-HCl reaction 2 h. 20 μL, 5 μmol / L of P2 solution was added, and 8 μL, 1 nmol / L of AUNPs were added. After 20 min, the temperature was added to 20 min, 1 μL, 500 mmol / L, pH = 5 citrate solution was added and oscillated at 37 ° C. 3 h was cultured, and the body P2 and the nano-gold were bonded through the Au-S key. The extra P2 chain was removed at 14,000 R / min to remove excess P2 chains, and the AUNPS @ P2 coupling can be obtained. 20 μL, 5 μmol / L of modified fluorescent group FAM was added to the AUNPS @ P2 coupled, and the medium temperature culture solution can be obtained to obtain a sensor working solution at 4 ° C.
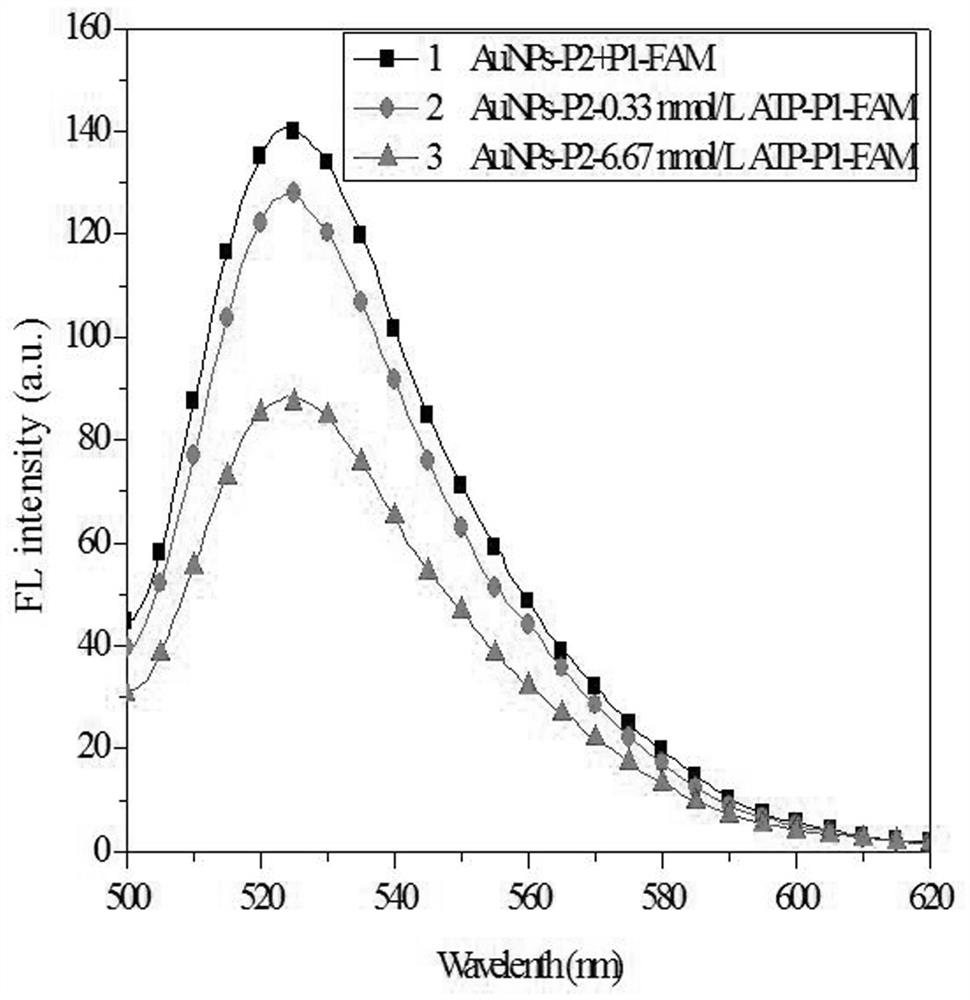
[0043]ATP was detected: 6 of the above working solutions were added, and 20 μl of concentrations were added to an ATP of 1 nmol / L, 15 nmol / L, 65 nmol / L, 100 nmol / L, 200 nmol...
Embodiment 2
[0046]The preparation method based on nano-gold split body sensor, the steps are as follows:
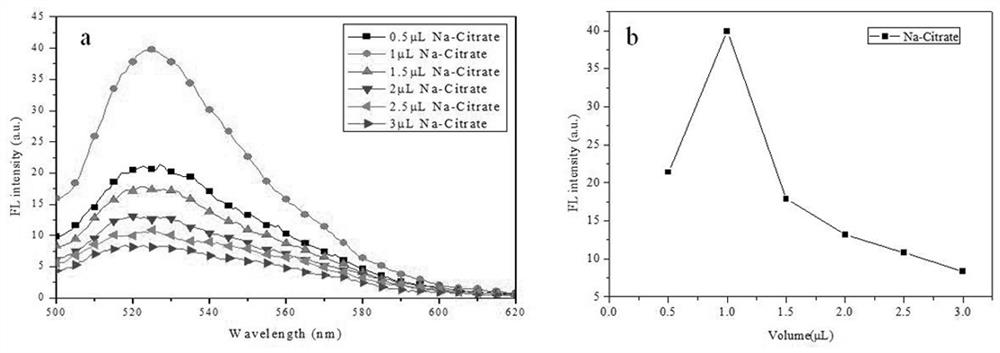
[0047]The preparation step of the present embodiment is the same as in Example 1, and only 0.5 μL, 1 μL, 1.5 μl, 2 μl of, 2.5 μL, 3 μl of 500 mmol / L, pH = 5, respectively. Sodium citrate solution, prepared a working solution different from the initial fluorescence intensity, such asimage 3 . With the increase of sodium citrate content, the initial fluorescence intensity of the working solution is presented and decreased before enhancement, such asimage 3 (B). This is because the amount of sodium citrate is too small, the suitable low pH solution environment cannot be formed, and the Au-S button is less in a short time, resulting in a naked state of the part of the AUNPS surface. Single-strand base P1 is easily adapt to the nano-gold surface, the distance between the FAM and AUNPS, thereby generating energy transfer from the FAM to the AUNPS surface, resulting in fluorescence quenching. As t...
Embodiment 3
[0049]The preparation method based on nano-gold split body sensor, the steps are as follows:
[0050]The preparation step of the present embodiment is the same as that of Example 1, maintaining the P2 concentration constant, only the volume of the AUNPS increases from 1 μL to 15 μL, and the AUNPS and P2 ratios have been prepared, and the initial fluorescence spectrum such asFigure 5 (A), peak changesFigure 5 (B). As can be seen from the figure, as the AUNPS concentration increases, the working solution is highly reduced to decrease. When the AUNPS volume is 8 μL, the fluorescent signal of the working solution is the strongest. It is understood that when the AUNPS concentration is less than 8 μl, the fluorescence intensity of the working solution increases with the concentration of AUNPS, which is due to the auNPS concentration is too low, a large number of P2 can be modified on a single AUNPS, and the AUNPS surface is almost P2 chain. Enclosed; at this time, the fluorescent signal in t...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com