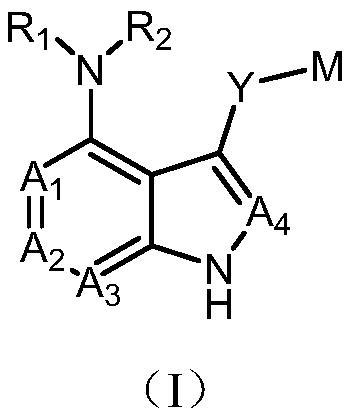
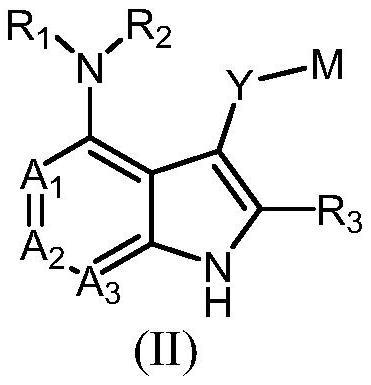
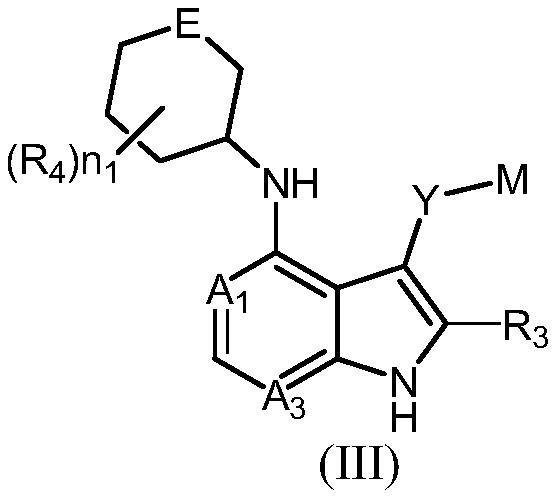
Compound serving as protein kinase inhibitor and application of compound
A compound and mixture technology, applied in the field of medicine, can solve problems such as unmet clinical needs and drug resistance
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0179]
[0180] Step 1: Synthesis of compounds 1-3
[0181] Compound 1-1 (11.232g, 72.2mmol), potassium carbonate (14.967g, 108.3mmol), 1-2 (8.154g, 86.6mmol), DMF (60mL) were added to the reaction flask, stirred at room temperature overnight, and TLC showed The response is complete. The reaction system was poured into water, extracted twice with ethyl acetate, the ethyl acetate layers were combined, washed twice with water, washed with saturated brine, anhydrous Na 2 SO 4 Dry and concentrate under reduced pressure to obtain 16.5 g of product 1-3, yield: 99%. The product was used directly in the next step without purification.
[0182] Step 2: Synthesis of compounds 1-4
[0183] Compound 1-3 (16.5g, 72mmol), KOH aqueous solution (220mL, 5M), and ethanol (45mL) were added to the reaction flask, and the temperature was raised to 100°C for overnight reaction. TLC showed that the raw materials were completely reacted. Cool the reaction system to 0°C, add concentrated hydro...
Embodiment 2
[0201]
[0202] Step 1: Synthesis of compound 2-2
[0203] Compound 2-1 (200mg, 1.31mmol) and THF (6mL) were added to the reaction flask, Lithium aluminum hydride (100mg, 2.63mmol) was added in batches to the reaction solution, and the reaction solution was stirred at room temperature overnight, and TLC showed that the reaction was complete . The reaction solution was cooled in an ice-water bath, and 200 mg of water was slowly added dropwise to quench the reaction. The resulting suspension was suction-filtered, the filter cake was washed with THF, and the filtrate was concentrated under reduced pressure to obtain 120 mg of product 2-2, yield: 74%. The product was used directly in the next step without purification.
[0204] Step 2: Synthesis of Compound 2
[0205] Compound 1-7 (50mg, 0.13mmol), 2-2 (32mg, 0.26mmol), TsOH (25mg, 0.13mmol) and n-butanol (2ml) were added to the reaction flask, and the reaction solution was reacted at 120°C for 2 hours. TLC showed the react...
Embodiment 3
[0209]
[0210] Compound 1 (60mg, 0.126mmol), potassium carbonate (53mg, 0.381mmol), absolute ethanol (1mL) and bromoethanol (16mg, 0.126mmol) were added to the reaction flask, the reaction mixture was heated to reflux and stirred for 1 hour, TLC Shows complete reaction. The reaction system was cooled to room temperature and directly concentrated to dryness under reduced pressure. Purified by preparative silica gel plate (DCM / MeOH=10 / 1) to obtain 10 mg of product 3, yield: 16%.
[0211] LC / MS: m / z=518.2[M+H]+.
[0212] 1H NMR(400MHz,d6-DMSO)δ1.33-1.38(2H,m),1.46-1.53(2H,m),1.61-1.70(2H,m),1.92-1.99(2H,m),3.54-3.67 (3H,m),3.78-3.82(2H,m),3.88-3.93(1H,m),4.15-4.16(1H,m),4.53(1H,t,J=5.7Hz),7.03(1H,dd ,J=8.5,2.5Hz),7.19-7.26(4H,m),7.47-7.51(2H,m),7.59-7.64(2H,m),8.25(1H,s),8.80-8.83(1H,m ),12.67(1H,brs).
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com