Injection filling material and preparation process
A technology of filling materials and raw materials, applied in the field of biotechnology, can solve the problems of hidden safety hazards, single function of hyaluronic acid and collagen, and achieve the effects of good biological safety, prolonging the effective time, and long-lasting effect.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
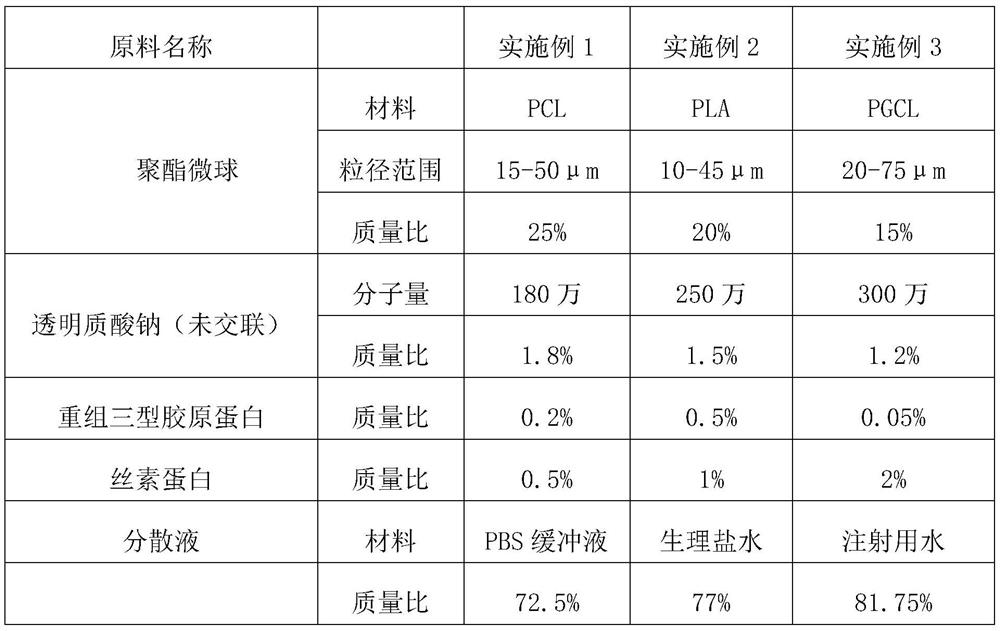
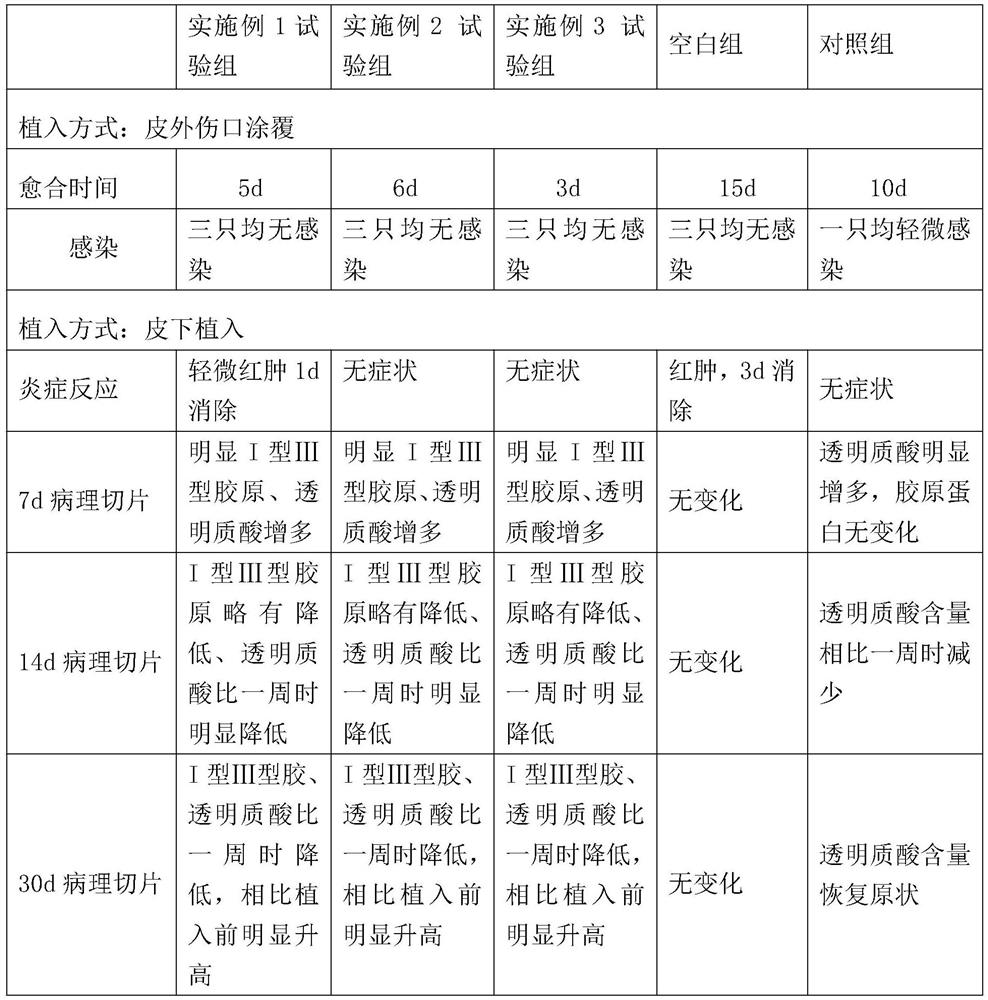
Embodiment 1-3
[0028] The preparation method of embodiment 1-3 injection filling material is:
[0029] S1. Weigh the polyester microspheres in proportion, add them to a certain amount of dispersion liquid, stir mechanically for 30min, and stir at a rate of 500rpm;
[0030] S2. Add the recombinant protein into the dispersion, and stir it magnetically for 40 minutes until it is completely dissolved;
[0031] S2. Add silk fibroin to the dispersed liquid after stirring, and keep stirring for 40 minutes;
[0032] S3. Place the dispersion under a homogenizer, and emulsify it at 10,000 rpm for 10 minutes to form a suspension;
[0033] S4. While vortex stirring in the suspension, add sodium hyaluronate powder, and continue to stir until the suspension is gelatinous and no vortex is formed on the surface;
[0034] S5. Stand still for 72-96 hours until the hyaluronic acid swells evenly;
[0035] S6, put the swollen sodium hyaluronate into an emulsification tank, and vacuumize while stirring to elim...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Particle size | aaaaa | aaaaa |
| Particle size | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com