Kras-G12C inhibitor heterocyclic compounds
A compound, -NH2 technology, applied in organic chemistry, drug combination, organic chemical methods, etc., can solve problems such as low activity, low blood drug concentration, and short half-life
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
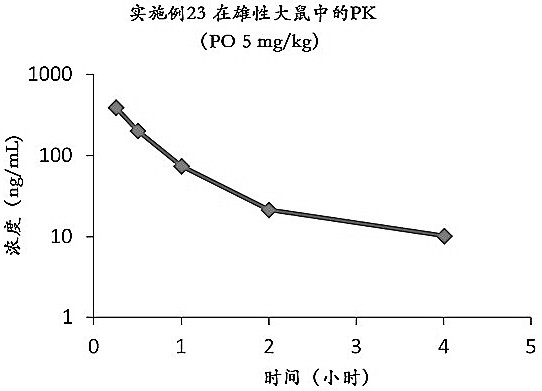
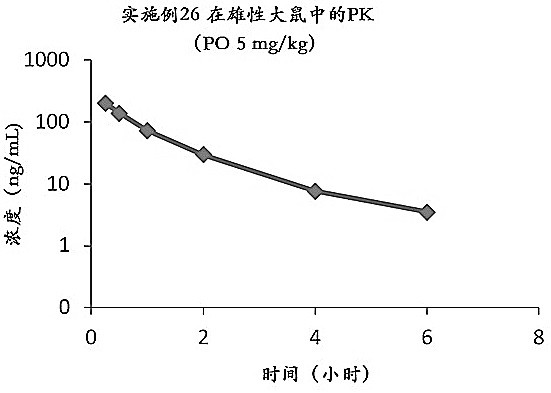
Image
Examples
Embodiment 1
[0069] 4-((S)-4-acryloyl-2-methylpiperazin-1-yl)-1-[2-(2,2-difluorocyclopropyl)-4-methylpyridin-3-yl ]-6-fluoro-7-(2-fluoro-6-hydroxyphenyl)pyridin[2,3-d]pyrimidin-2(1H)-one
[0070]
[0071] Step A: 1-Methyl-2-nitro-3-vinylpyridine
[0072]
[0073] Under nitrogen protection, 2-bromo-3-nitro-4-methylpyridine (1 g), Pd(dppf)Cl 2 (337 mg), potassium carbonate (2 g), and potassium ethylene trifluoroborate (0.94 g) were dissolved in a mixed solvent of 1,4-dioxane / water (35 mL / 5 mL), and the system was heated to 120°C Stir overnight. Cool to room temperature, concentrate under reduced pressure, add ethyl acetate to dissolve the residue, filter with celite, concentrate the filtrate under reduced pressure, and separate the residue by silica gel column chromatography (EtOAc / PE = 1 / 5) to obtain the product (603 mg).
[0074] Step B: 2-(2,2-Difluorocyclopropyl)-4-methyl-3-nitropyridine
[0075]
[0076] Under nitrogen protection, 1-methyl-2-nitro-3-vinylpyridine (100 mg), ...
Embodiment 2
[0114] 4-((S)-2-acryloyl-2-methylpiperazin-1-yl)-1-[2-(difluoromethyl)-4-methylpyridin-3-yl]-6-fluoro -7-(2-fluoro-6-hydroxyphenyl)pyrido[2,3-d]pyrimidin-2(1H)-one
[0115]
[0116] Step A: 2,4-Dimethyl-3-nitropyridine
[0117]
[0118] Under nitrogen protection, 2-bromo-4-methyl-3-nitropyridine (2.4 g), trimethylboroxane (2.8 g), Pd(dppf)Cl 2 (0.4 g) and potassium carbonate (4.6 g) dispersed in 1,4-dioxane (30 mL) and H2 O (5 mL) in a mixed solvent, then heated to 120 °C and refluxed for 3 hours. Cooled to room temperature, the reaction mixture was filtered with celite, the filtrate was extracted with ethyl acetate and water, the organic phases were combined, concentrated under reduced pressure, and the residue was separated by silica gel column chromatography (EtOAc / PE = 1 / 5) to obtain the product ( 1.6g).
[0119] 1 H NMR (400 MHz, CDCl 3 ), 8.42 (d, J = 4.8 Hz, 1H), 7.08 (d, J = 4.8Hz, 1H), 2.52 (s, 3H), 2.31 (s, 3H).
[0120] Step B: 4-Methyl-3-nitrobenzaldehy...
Embodiment 3
[0157] 4-((S)-2-acryloyl-2-methylpiperazin-1-yl)-1-[2-(1,1-difluoroethyl)-4-methylpyridin-3-yl] -6-fluoro-7-(2-fluoro-6-hydroxyphenyl)pyrido[2,3-d]pyrimidin-2(1H)-one
[0158]
[0159] Step A: 2-(1-Ethoxyvinyl)-4-methyl-3-nitropyridine
[0160]
[0161] Under nitrogen protection, 2-bromo-4-methyl-3-nitropyridine (1.1 g), tributyl(1-ethoxyvinyl)tin (2.2 g) and Pd(dppf)Cl 2 (0.2 g) was dispersed in 1,4-dioxane (30 mL), then heated to 120°C and refluxed for 24 hours. Cool to room temperature, add saturated KF aqueous solution (50 mL) and continue stirring for 1 hour, then filter the reaction mixture with Celite, extract the filtrate with ethyl acetate (50 mL×3), combine the organic phases, concentrate under reduced pressure, and the residue The product (0.9 g) was isolated by silica gel column chromatography (EtOAc / PE = 1 / 5).
[0162] 1 H NMR (400 MHz, CDCl 3 ), 8.49 (d, J = 5.2 Hz, 1H), 7.19 (d, J = 5.2Hz, 1H), 5.15 (d, J = 2.8 Hz, 1H), 4.49 (d, J = 2.8 Hz, 1H), 3.87...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com