Lewis acid-base bifunctional catalyst and preparation method and application thereof
A dual-functional catalyst and catalyst technology, which is applied in the direction of catalyst activation/preparation, catalytic reaction, and carboxylic acid nitrile preparation, can solve the problems of uncontrollable MOFs assembly process, single catalyst function, and large unpredictability, and achieve excellent catalysis High performance, high recycling rate, and the effect of less catalyst consumption
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0031] Embodiment 1: the synthesis of Lewis acid-base bifunctional catalyst
[0032] (1) Weigh according to the molar ratio of 2,4,6-tris(4-pyridyl)-1,3,5-triazine to trimesic acid at 1:1, and mix to form a mixed compound;
[0033] (2) Weigh nickel sulfate hydrate metal salt according to the molar ratio of nickel sulfate hydrate and 2,4,6-tris(4-pyridyl)-1,3,5-triazine in step (1) in a ratio of 1:1 ;
[0034] (3) Nickel sulfate hydrate and the mixture were stirred in DMF / EtOH / H 2 O (volume ratio is 4:1:2) mixed in a mixed solvent, configured as a precursor solution;
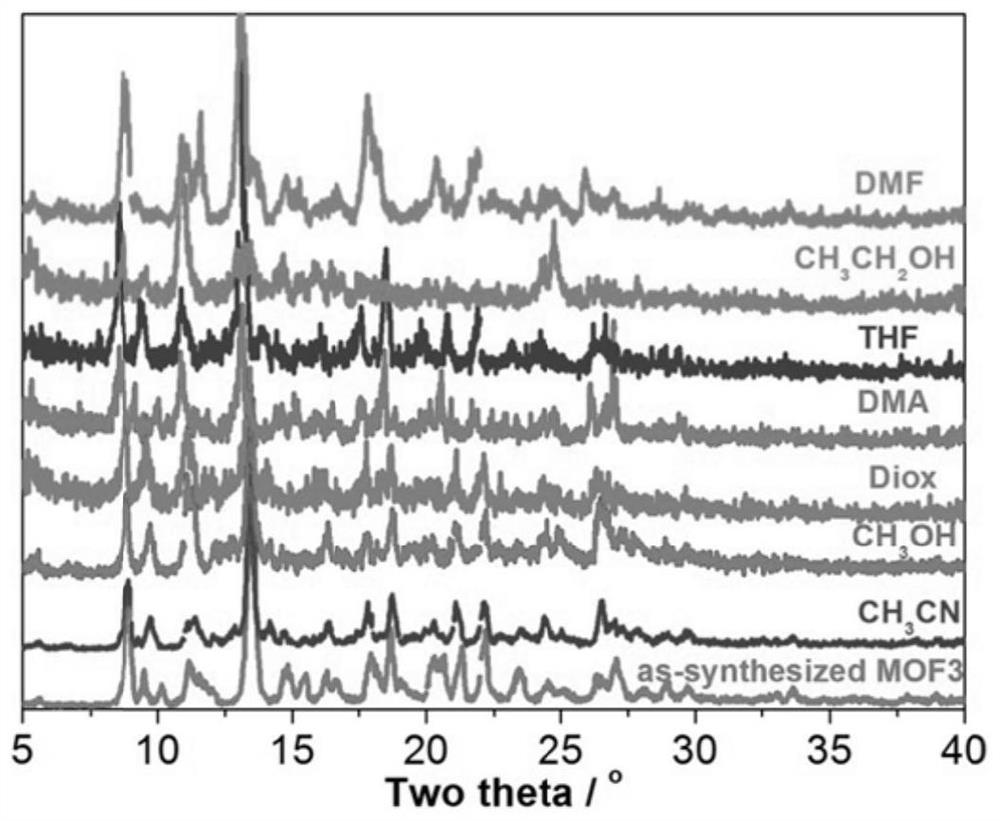
[0035] (4) Transfer the precursor solution obtained in (3) into a hydrothermal kettle with a volume ratio of 1 / 3, perform solvothermal reaction at 100° C. for 72 hours, suction filter, wash, and dry to obtain a catalyst.
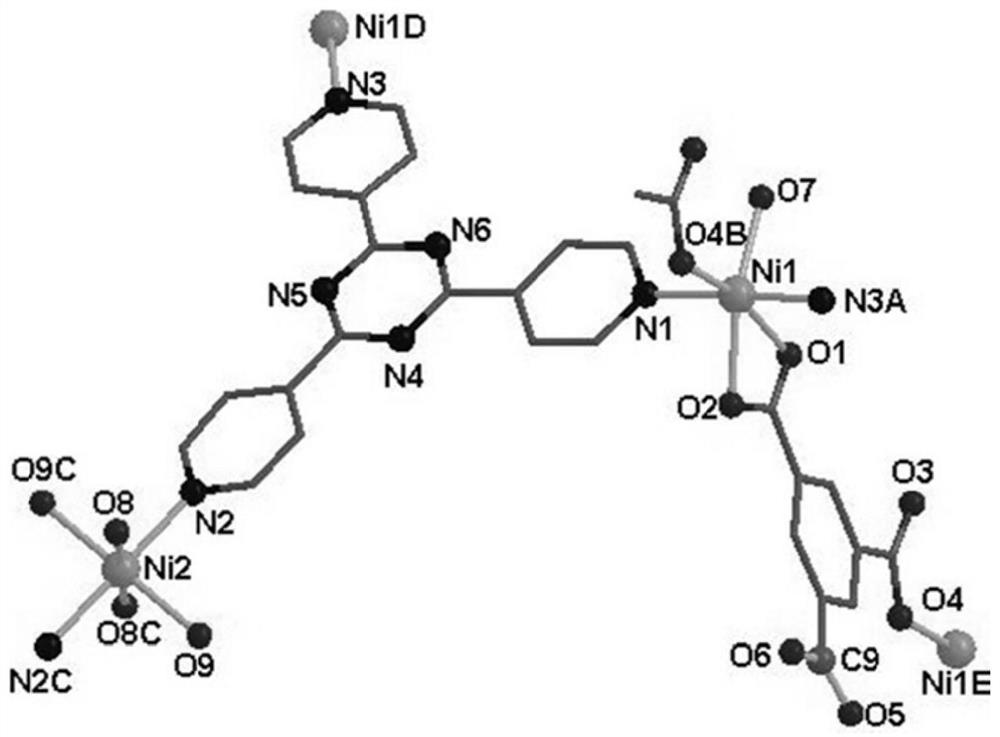
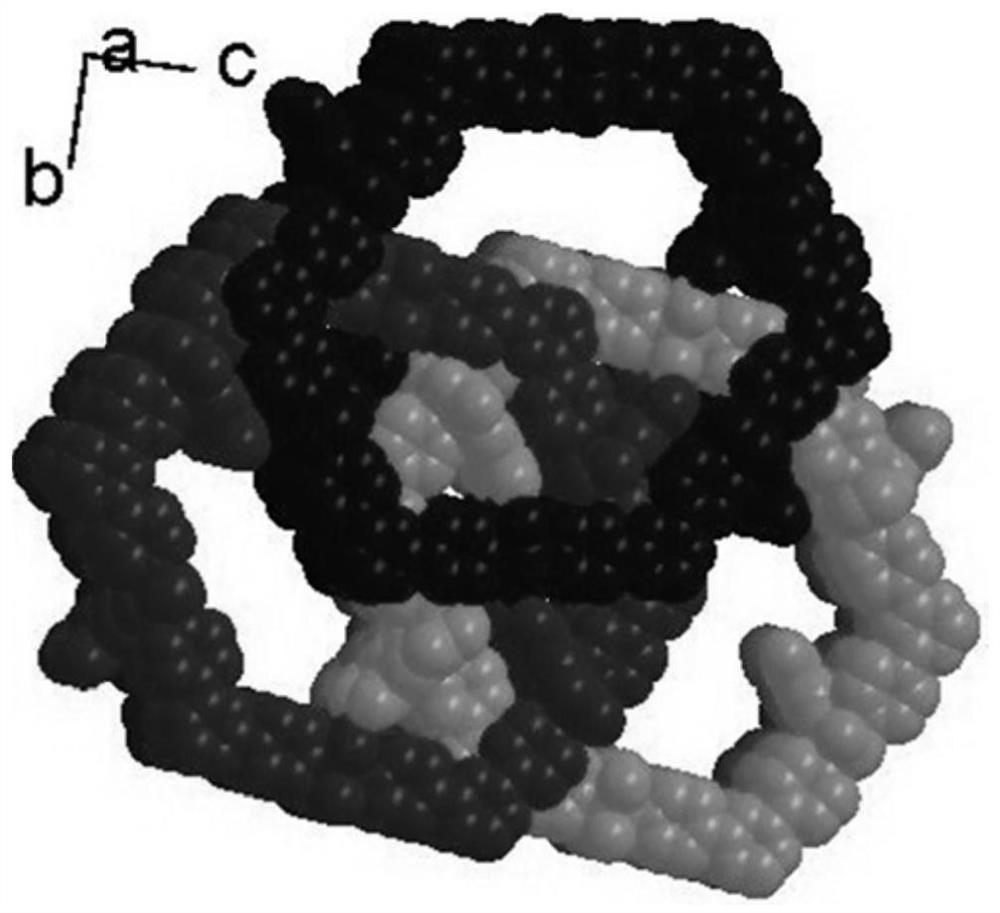
[0036] The catalyst obtained is composed of 2,4,6-tris(4-pyridyl)-1,3,5-triazine and trimesic acid to form a mixed body, with Ni(II) as the central metal ion, the mixed Ligands and Ni(II) ass...
Embodiment 2
[0039] Embodiment 2: the synthesis of Lewis acid-base bifunctional catalyst
[0040] This example is basically the same as Example 1, except that the solvothermal reaction in the step (4) of this example is carried out at a temperature of 80° C. for 48 hours.
Embodiment 3
[0041] Embodiment 3: the synthesis of Lewis acid-base bifunctional catalyst
[0042] This example is basically the same as Example 1, except that in step (2) of this example, nickel sulfate hydrate and 2,4,6-tris(4-pyridyl)-1,3 in (1) , The molar ratio of 5-triazine is 2:1, and the hydrated nickel sulfate metal salt is weighed, and the solvothermal reaction in step (4) of this embodiment is carried out at a temperature of 120° C. for 60 hours.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com