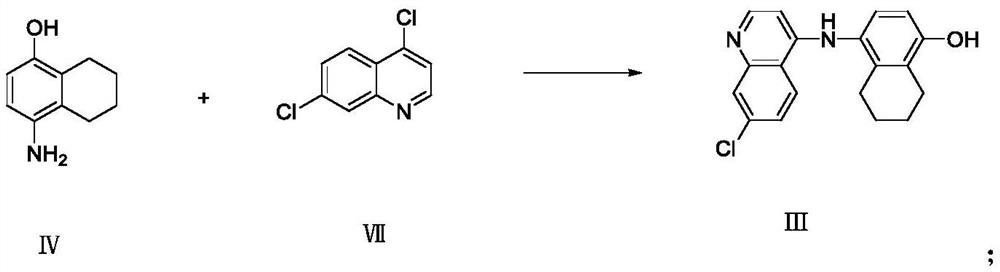
Preparation method of naphthoquine phosphate
A technology of naphthoquine phosphate and phosphoric acid, which is applied in the field of medicine, can solve the problems of low reaction conversion rate, unfavorable production, large alloy consumption, etc., and achieves the effects of easy availability of raw materials, solving process safety problems, and less environmental pollution
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
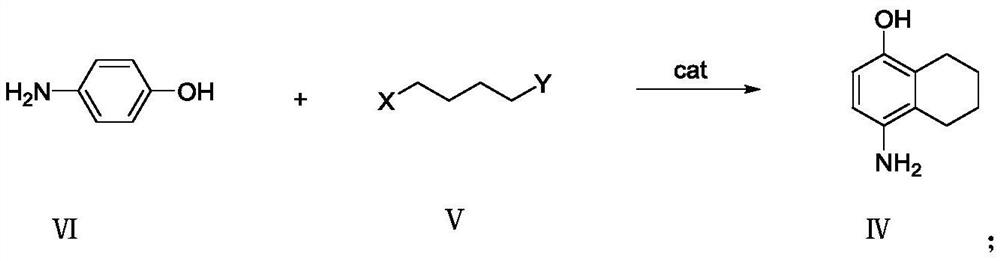
[0022] Example 1 Preparation of 4-amino-5,6,7,8-tetrahydro-1-naphthol formula IV
[0023] Add 54.6g of p-aminophenol to a 1L reaction flask, add 327.6g of dichloromethane and stir, cool to 0°C~5°C, add 20g of anhydrous aluminum chloride in batches, and slowly add 63.5g of 1,4-dichlorobutane dropwise After the dropwise addition, the temperature was raised to 25°C to 35°C to react, TLC followed the reaction progress, the reaction was complete, the reaction solution was poured into ice water, 50% sodium hydroxide aqueous solution was added, the pH was adjusted to 8-9, the layers were separated, and the organic layer was After washing with water for several times, the solvent was removed under reduced pressure to obtain 75.6 g of the product with a content of 89.5% and a yield of 82.9%.
Embodiment 2
[0024] Example 2 Preparation of 4-amino-5,6,7,8-tetrahydro-1-naphthol formula IV
[0025] Add 54.6g of p-aminophenol to a 1L reaction flask, add 327.6g of dichloromethane and stir, cool with ice water to 0°C-5°C, add 30g of anhydrous aluminum trichloride in batches, slowly add 108.0g of 1,4-dibromo After the addition of butane, the temperature was raised to 25°C to 35°C to react, TLC followed the reaction, and the reaction was complete. Pour the reaction solution into ice water, add 50% sodium hydroxide aqueous solution, adjust the pH to 8-9, and separate layers. The organic layer was washed several times with water, and the solvent was removed under reduced pressure to obtain 79.2 g of the product with a content of 87.5% and a yield of 84.9%.
Embodiment 3
[0026] Example 3 Preparation of 4-amino-5,6,7,8-tetrahydro-1-naphthol formula IV
[0027] Add 54.6g of p-aminophenol into a 1L reaction flask, add 382.2g of dichloromethane and stir, cool with ice water to 0°C-5°C, add 97.3g of anhydrous ferric chloride in batches, slowly add 63.5g of 1,4-dichloromethane Chlorobutane, after the dropwise addition, heat up to 25°C to 35°C to react, TLC to track the reaction progress, the reaction is complete, pour the reaction solution into ice water, add 50% sodium hydroxide aqueous solution, adjust the pH to 8-9, and separate layers , the organic layer was washed several times with water, and the solvent was removed under reduced pressure to obtain 65.9 g of the product with a content of 77.8% and a yield of 62.8%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com