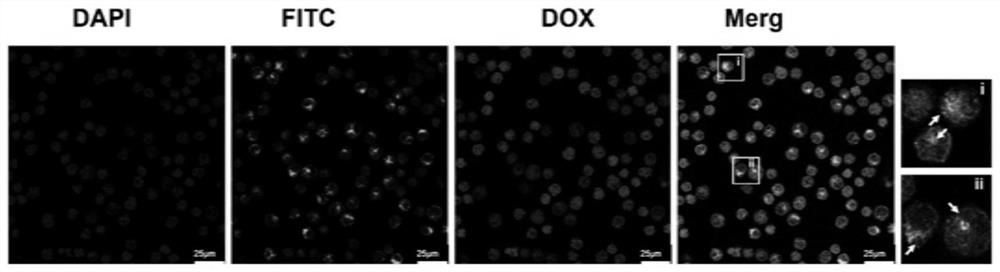
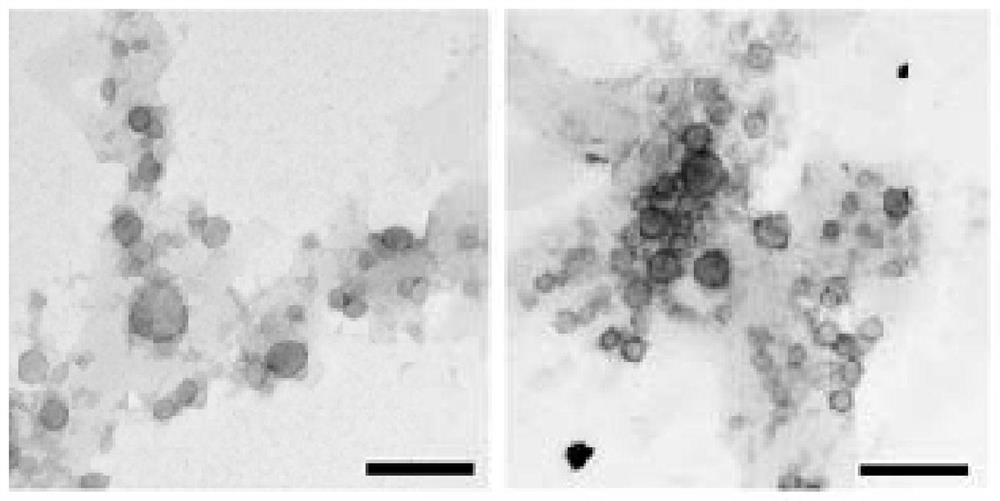
A kind of exosome targeting lymphoma cells and its preparation method and application
A technology of lymphoma cells and exosomes, which is applied to medical preparations with non-active ingredients, medical preparations containing active ingredients, and pharmaceutical formulas, etc., can solve the problems of accurate detection and low drug targeting efficiency, and achieve High drug loading rate, prolonged circulation time in vivo, and good biocompatibility
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0043] A CD22-F (ab') carrying doxorubicin 2 A method for preparing an exosome-targeted drug-carrying system of antibody fragments, the method comprising the following steps:
[0044] Step 1: Select 293T cells of the human kidney epithelial cell line as exosome-derived cells, and replace the culture medium of exosome-derived cells with serum-free medium 12-48 hours before extracting exosomes. After culturing for 24-48 hours, collect the cell suspension;
[0045] Step 2: Centrifuge the cell suspension (300g, 10min, 4°C), discard the cells and debris, take the supernatant and centrifuge again (2000g, 10min, 4°C), take the supernatant and centrifuge again (12000g, 30min, 4°C ), the supernatant after the third centrifugation was transferred to a 100kD ultrafiltration tube to balance, and concentrated by centrifugation (4000g, 15min, 4°C). Centrifuge the concentrate at an ultrahigh speed (100,000g, 90min, 4°C), discard the supernatant, resuspend and wash, then centrifuge at the s...
Embodiment 2
[0058] A CD22-F(ab') carrying cyclophosphamide 2 A method for preparing an exosome drug-carrying system of an antibody fragment, the method comprising the following steps:
[0059] Step 1: Select 293T cells of the human kidney epithelial cell line as exosome-derived cells, and replace the culture medium of exosome-derived cells with serum-free medium 12-48 hours before extracting exosomes. After culturing for 24-48 hours, collect the cell suspension;
[0060] Step 2: Centrifuge the cell suspension (300g, 10min, 4°C), discard the cells and debris, take the supernatant and centrifuge again (2000g, 10min, 4°C), take the supernatant and centrifuge again (12000g, 30min, 4°C ), the supernatant after the third centrifugation was transferred to a 100kD ultrafiltration tube to balance, and concentrated by centrifugation (4000g, 15min, 4°C). Centrifuge the concentrate at an ultrahigh speed (100,000g, 90min, 4°C), discard the supernatant, resuspend and wash, then centrifuge at the same...
PUM
| Property | Measurement | Unit |
|---|---|---|
| diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com