Methods and systems for selection and treatment of patients with inflammatory diseases
An inflammatory disease, active technology, applied in the field of selection and treatment of inflammatory disease patients and systems, can solve the problem of less personalized therapy
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[1246] Example 1: Identification of Genotypes
[1247] IBD patients were recruited at the Cedars-Sinai Inflammatory Bowel Disease Centers. The diagnosis of each patient was based on standard endoscopic, histological, and radiographic features. Blood samples were collected from patients at enrollment. Blood samples were also collected from individuals without IBD. All samples collected were genotyped at Cedars-Sinai Medical Center using Illumina whole genome arrays following the manufacturer's protocol (Illumina, San Diego, CA). Markers / SNPs were excluded from analysis if: there was a deviation in the Hardy–Weinberg balance in controls, p ≤ 0.0001; there was >2% defect in the SNP, and the minor allele frequency was 0.25) and excluded from analysis (PLINK). Generate race proportion estimates for all individuals using a mixture. Only subjects identified as Caucasian by admixture (proportion <0.75) were included in the analysis.
[1248] Multiple studies involving inflammator...
Embodiment 2
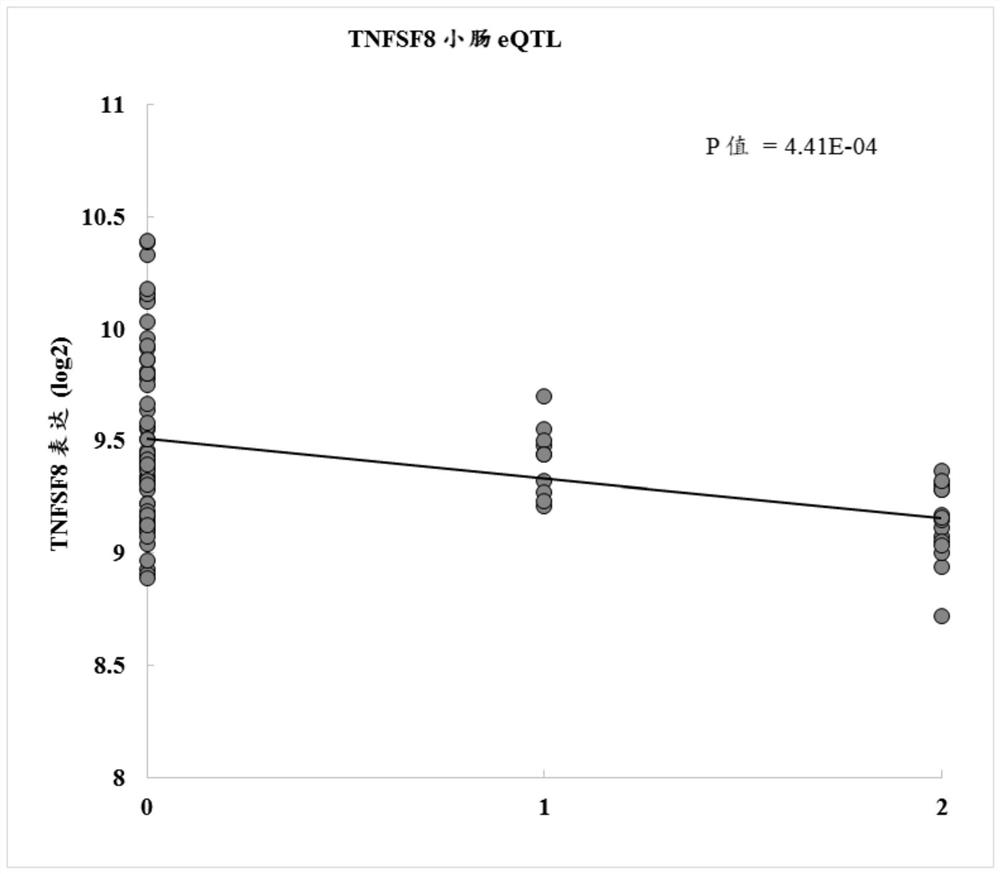
[1249] Example 2: eQTL in small bowel resection identifies polymorphisms as functionally associated with CD30L protein expression
[1250] Eighty-five Caucasian patients with Crohn's disease (CD) who underwent small bowel resection were recruited at the Cedars-Sinai Inflammatory Bowel Disease Centers. The diagnosis of each patient was based on standard standard endoscopic, histological, and radiographic features. Patients were selected on the basis of being diagnosed with CD and having undergone small bowel resection for the disease. Tissue biopsy samples were collected from uninvolved tissue sections resected from the small bowel after surgery. Expression quantitative trait locus mapping (eQTL) was performed on these samples. Table 13 provides polymorphisms associated with reduction of CD30. Table 14 provides polymorphisms associated with increased CD30. In tissue samples, a negative beta value indicates a decrease in gene expression, while a positive beta value indicates...
Embodiment 3
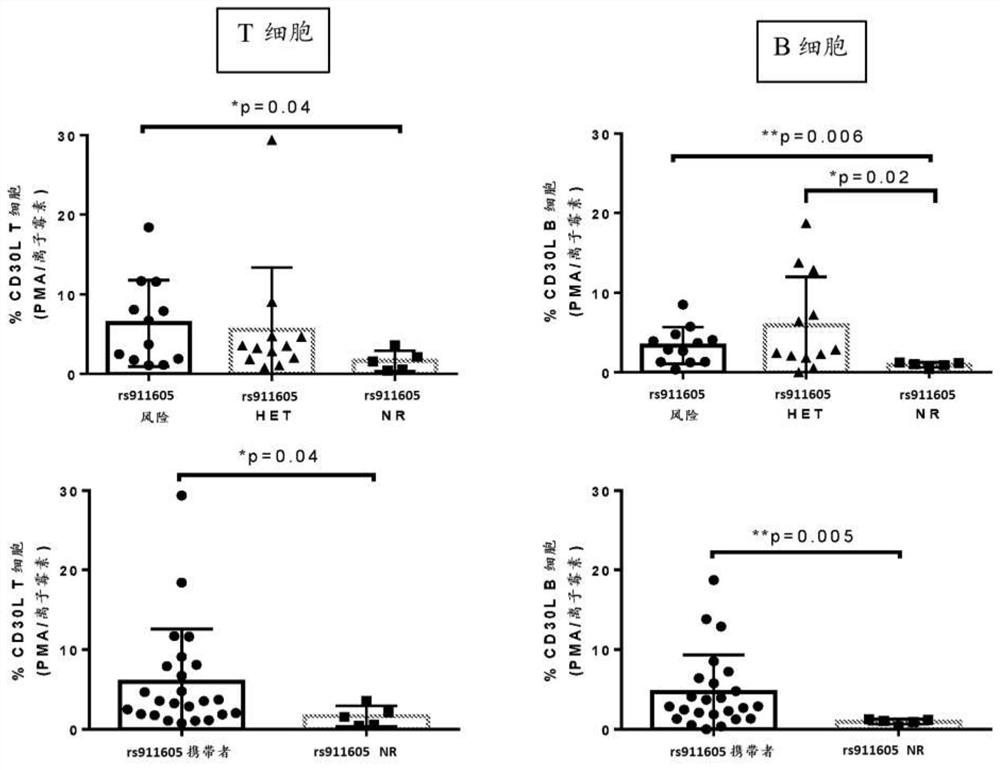
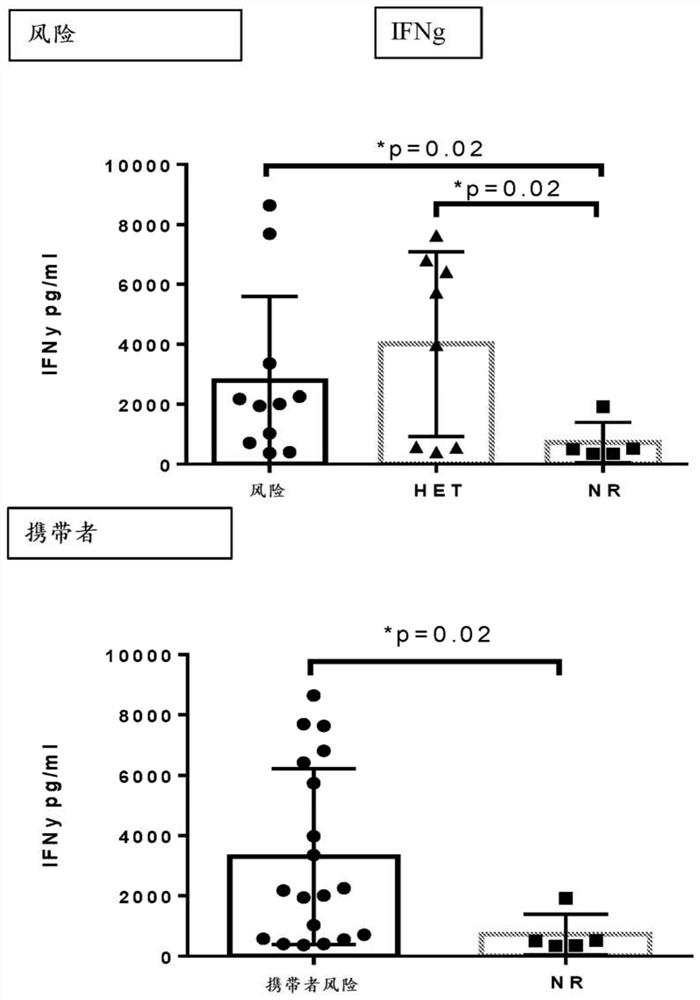
[1251] Example 3. Polymorphic expression affects the expression and function of CD30L and pro-inflammatory cytokines in peripheral blood
[1252] Crohn's disease (CD) patients were recruited at the Cedars-Sinai Inflammatory Bowel Disease Centers. The diagnosis of each patient was based on standard endoscopic, histological, and radiographic features. Patients were selected on the basis of being diagnosed with CD. Peripheral blood samples were collected from patients at enrollment. Peripheral blood mononuclear cells (PBMC) were isolated on standard Ficoll-Hypaque density gradients. PBMCs were stimulated in vitro for 72 hours under conditions that upregulated CD30L (phorbol 12-myristate 13-acetate (PMA) and ionomycin) or CD30 (anti-CD3 antibody and anti-CD28 antibody). Supernatants were collected at 6, 24 and 72 hours for cytokine analysis, and cells were collected after 72 hours and analyzed for CD30 and CD30L expression by flow cytometry. In subjects carrying (homozygous or...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Body mass index | aaaaa | aaaaa |
| Weight | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com