Method for preparing chenodeoxycholic acid from seal cholic acid
A technology of chenodeoxycholic acid and cholic acid, applied in the field of chenodeoxycholic acid, can solve problems such as waste, achieve the effects of avoiding biological waste pollution, alleviating insufficient supply, good economic value and application prospects
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
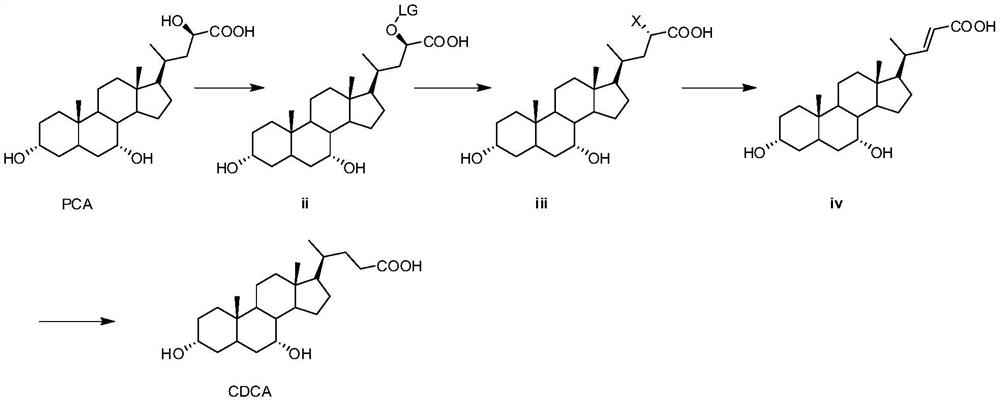
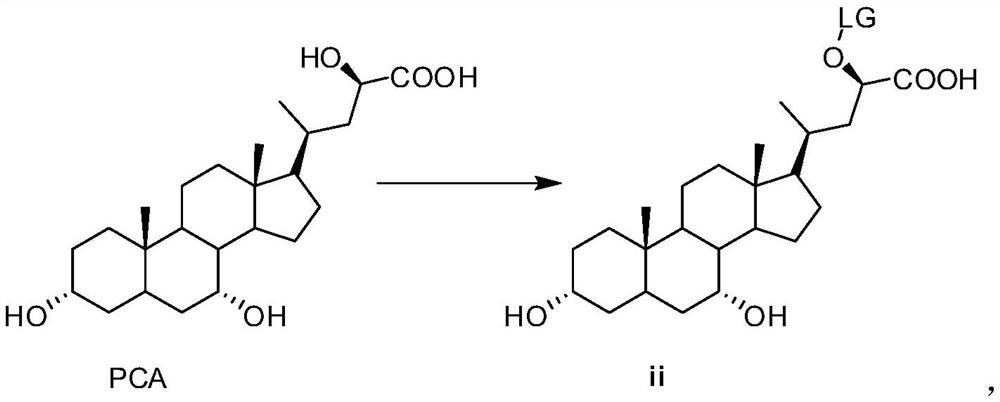
[0040] (1) Preparation of compound ii
[0041] N 2 Under protection, PCA (600 g, 1.47 mol) and N,N-diisopropylethylamine (341.6 g, 2.64 mol) were added into 12 L of dichloromethane with stirring started. The temperature was lowered to 0-5°C, and methylsulfonyl chloride (185 g, 1.61 mol) was added dropwise. After the dropwise addition, continue to keep stirring for 8h. After adding 2L of water and stirring, the liquid was separated, and the organic phase was washed once with 3.1L of saturated ammonium chloride aqueous solution, dried by adding anhydrous sodium sulfate, and concentrated to obtain 764.7g of compound ii.
[0042] 1 HNMR (400MHz, DMSO) δ12.61(s, 1H), 5.42(m, 1H), 4.34(d, 1H), 4.16(d, 1H), 3.60(m, 1H), 3.17(m, 1H), 3.10(s, 3H), 1.15-1.8(m, 24H), 1.09(d, 3H), 0.99(s, 3H), 0.59(s, 3H)
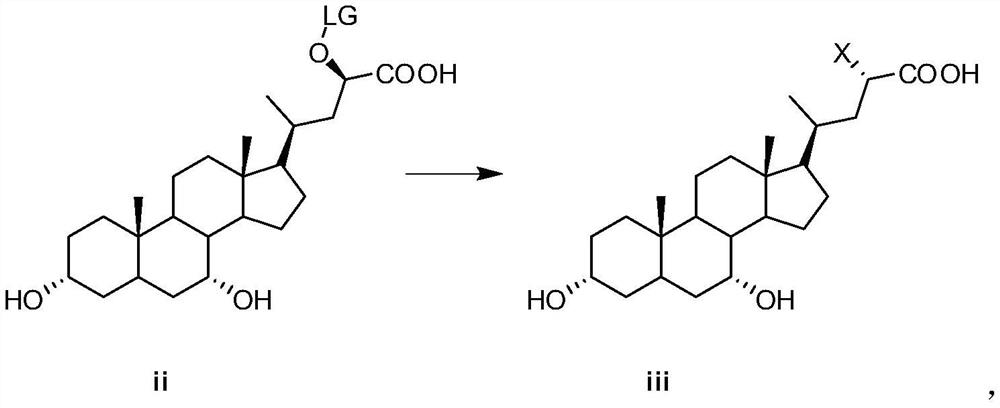
[0043] (2) Preparation of compound iii
[0044] Compound ii (764.7 g, 1.47 mol) and lithium bromide monohydrate (462.4 g, 4.41 mol) obtained in the previous step were added to 7....
Embodiment 2
[0054] (1) Preparation of compound ii
[0055] N 2 Under protection, PCA (0.97 kg, 2.37 mol) and diazabicyclo (722 g, 4.74 mol) were added into 15 L of tetrahydrofuran and stirred. Cool down to 0-5°C, p-toluenesulfonyl chloride (472.81g, 2.48mol). After the dropwise addition, continue to keep stirring for 10 h. Add 2L of ethyl acetate and 5L of water for extraction and separation. The organic phase was washed once with 5 L of saturated aqueous ammonium chloride solution, dried by adding anhydrous sodium sulfate, and then concentrated to obtain 1.41 kg of compound ii.
[0056] 1 HNMR (400MHz, DMSO) δ12.84(s, 1H), 7.89(d, 2H), 7.38(d, 2H), 5.44(m, 1H), 4.36(d, 1H), 4.17(d, 1H), 3.65(m, 1H), 3.21(m, 1H), 2.43(s, 3H), 0.9-1.9(m, 24H), 1.12(d, 3H), 0.98(s, 3H), 0.59(s, 3H)
[0057] (2) Preparation of compound iii
[0058] Compound ii (1.41kg, 2.37mol) and sodium bromide (610.1g, 5.93mol) were added to 10L acetone and refluxed for 24h. After concentration under reduced press...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com