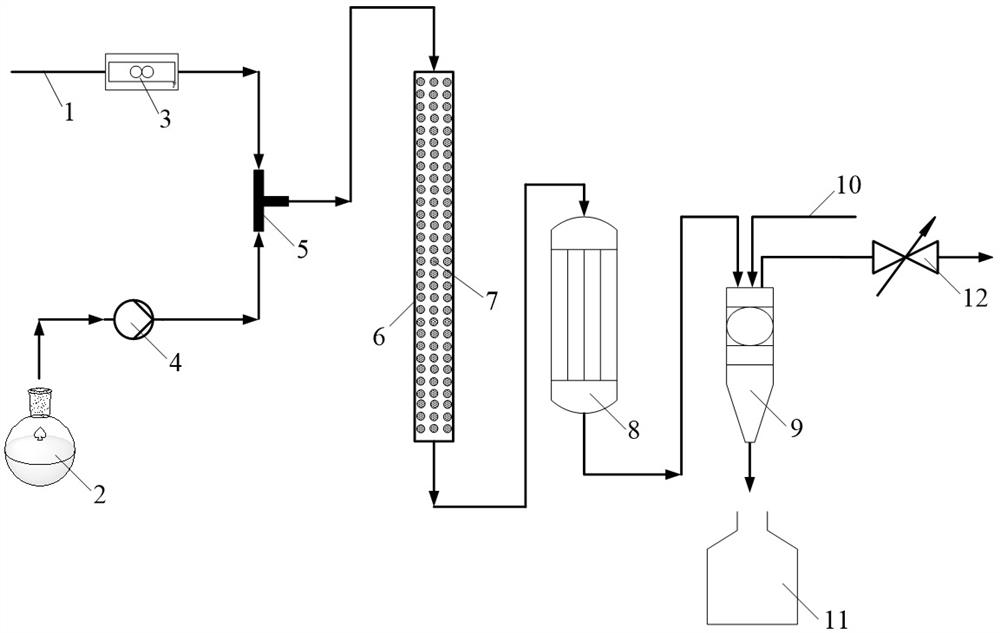
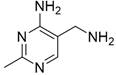
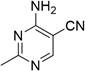
Micro-reaction system and method for continuously preparing 2-methyl-4-amino-5-aminomethylpyrimidine by using same
A technology of aminomethylpyrimidine and micro-reaction, applied in chemical instruments and methods, chemical/physical/physicochemical reactors, metal/metal oxide/metal hydroxide catalysts, etc., can solve the problem of low process efficiency, synthetic Short route, high reaction pressure and other problems, to achieve the effect of improving efficiency, time-space yield, small liquid holding capacity, and high space-time efficiency
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0060] 1. Preparation of modified Raney nickel catalyst
[0061] Disperse 40 g of 20-40 mesh Raney nickel in 100 g of water, add 5% formalin solution of Raney nickel weight, stir at 25°C for 2 hours under nitrogen protection, then filter and wash with deionized water three times , that is, the modified Raney nickel catalyst is prepared, and finally stored in water.
[0062] 2. Catalytic hydrogenation reaction
[0063] The Raney nickel catalyst modified in this example above was filled in a tubular microchannel reactor (length 20 cm, inner diameter 1 cm). Take 5 g of ammonia water (25 wt.%) and 200 ml of methanol to prepare a methanol solution of ammonia water, then add 2-methyl-4-amino-5-cyanopyrimidine (2 g, 0.015 mol) to the above ammonia water Methanol solution was prepared as a substrate liquid, and then the substrate liquid and hydrogen gas were delivered to the T-type micro-mixer at the same time. The molar ratio of 2-methyl-4-amino-5-cyanopyrimidine to hydrogen is 1:...
Embodiment 2
[0065] This example is the same as Example 1, the only difference being that the preparation of the modified Raney nickel catalyst in this example is 60-80 mesh Raney nickel. In this example, the substrate 2-methyl-4-amino-5-cyanopyrimidine was completely converted, and the yield of the product 2-methyl-4-amino-5-aminomethylpyrimidine was 100%, and the purity was greater than 99%.
Embodiment 3
[0067] This example is the same as Example 2, the only difference is that in the catalytic hydrogenation reaction experiment in this example, the reaction of the mixed reaction material formed after the substrate liquid and hydrogen are mixed in a T-type micro-mixer in a microchannel reactor The time is about 1.2 minutes. In this example, the substrate 2-methyl-4-amino-5-cyanopyrimidine was completely converted, and the yield of the product 2-methyl-4-amino-5-aminomethylpyrimidine was 100%, and the purity was greater than 99%.
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle size | aaaaa | aaaaa |
| length | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com