Application of bacillus marinus sp. BS11 exopolysaccharide in preparation of anti-inflammatory drugs
A marine bacillus and exopolysaccharide technology, applied in the field of biomedicine, can solve the problems that have not yet been reported on the anti-inflammation of exopolysaccharides
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0026]The marine Bacillus sp.BS11 exopolysaccharide used in the present invention is obtained according to the method disclosed in the invention patent CN107988105A. The marine Bacillus sp.BS11 obtained. Specifically: pick the pure culture of marine Bacillus sp.BS11 and inoculate it into 5 mL of liquid 2216E medium, culture it with shaking at 28°C and 160 rpm for 24 hours, and prepare the seed fermentation liquid. Inoculate 500 mL of 2216E culture medium (with 1% sucrose) with 0.1% seed fermentation broth of Bacillus sp.BS11, place on a shaker at 28° C., and shake at 160 rpm for 48 hours. Centrifuge the fermentation broth at 8000rpm for 20min to remove bacteria, add 95% ethanol solution slowly at 3 times the volume of the supernatant and overnight at 4°C, centrifuge at 8000rpm for 20min, take the precipitate, and add water to dissolve the precipitate. After the precipitate was dissolved, the protein was removed by the Sevage method, and the solution was dialyzed using a dialys...
Embodiment 2
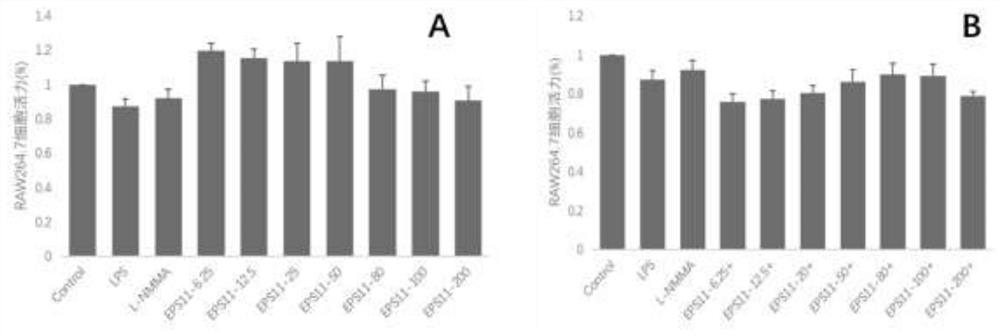
[0028] Effects of EPS11 administered in vitro on the proliferation activity of RAW264.7 cells
[0029] experimental method
[0030] Take the RAW264.7 cells in the logarithmic phase of growth, pipette to make a single cell suspension, and count the RAW264.7 cells according to 5×10 5 cells / mL were cultured in a 96-well plate, and when the cells grew to about 50%, 100 μL of FBS-free medium was used to replace the old medium, and starvation was performed for 12 hours. Then replace the old medium with 100 μL blank or medium containing different concentrations of EPS11, and continue to culture for 24 hours. Add 20 μL of MTT solution with a concentration of 5 mg / mL to each well, continue to incubate for 4 h, discard the supernatant, add 150 μL DMSO to each well, shake gently at room temperature for 10 min to fully dissolve the crystals, and measure the absorbance at a wavelength of 490 nm.
[0031] Cell survival rate=(OD value of experimental group / OD value of blank control group)×...
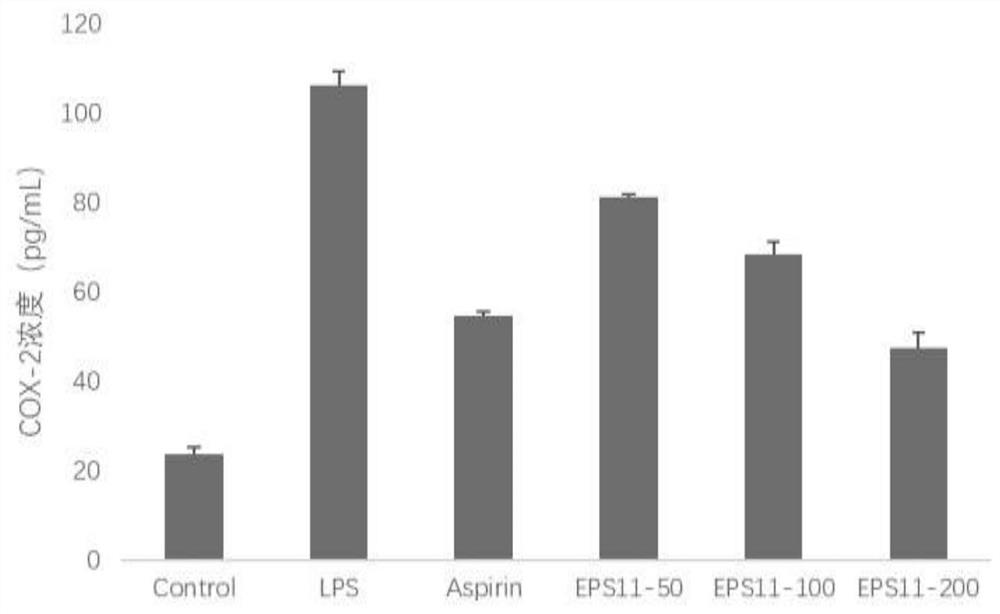
Embodiment 3
[0034] In vitro administration of EPS11 inhibited the secretion of NO secreted by RAW264.7 cells.
[0035] experimental method
[0036] 1. RAW264.7 cell culture
[0037] The mouse mononuclear macrophage cell line RAW264.7 was purchased from the Cell Resource Center of Shanghai Institutes of Biological Sciences, Chinese Academy of Sciences. RAW264.7 cells were cultured in DMEM high-glucose medium containing 10% fetal bovine serum in an incubator at 37°C with 5% CO2 saturated humidity. Change the medium every other day to ensure adequate nutrition. Observe the cell density every day. When the cell adherent growth reaches about 80%, it usually takes 2-3 days for subculture or other operations.
[0038] When subculture, carefully discard the original culture solution, rinse the bottle wall with PBS for 5 minutes, so that the cells are easy to blow off, transfer the new culture solution into the culture bottle, use a pipette gun to draw the culture solution and blow it carefully...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Molecular weight | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com