Application of compound ZJU-37 in preparation of medicines for preventing and/or treating liver diseases
A technology of ZJU-37, liver disease, applied in the field of preparation of drugs for the prevention and/or treatment of liver disease, can solve problems such as unclear specific mechanism, and achieve the effect of obvious liver protection and treatment effects and obvious social benefits
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0026]Example 1 Preparation of compound ZJU-37 solution
[0027]Experimental method: Compound ZJU-37 was prepared as a storage solution of compound ZJU-37 after DMSO was used to help dissolve, and was diluted with PBS to prepare a solvent for compound ZJU-37 before injection.
Embodiment 2
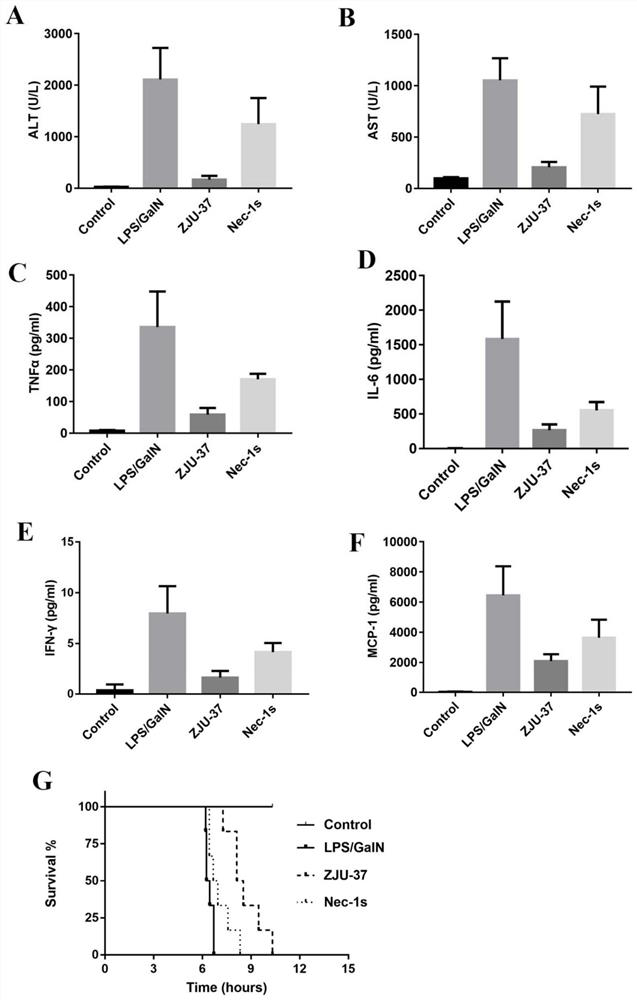
[0028]Example 2 Study of Compound ZJU-37 and Nec-1s on LPS / GalN-induced acute liver failure, liver injury and systemic inflammation in mice
[0029]experimental method:
[0030]Animal models are research tools to verify whether new therapeutic drugs and new therapeutic methods are effective. The animal models currently used internationally to simulate liver failure include drug-induced liver failure models (para-acetaminophen model, LPS / GalN model, sulfur Acetamide model, carbon tetrachloride model, etc.), acute liver ischemia model, partial hepatectomy model, etc. Among them, the LPS / GalN model, because of its good reproducibility, insignificant extrahepatic toxicity, and liver injury performance close to clinical advantages, is an ideal liver failure animal model.
[0031]Six-week-old male C57BL / 6 mice (purchased from Nanjing Institute of Biological Medicine) were randomly divided into normal control group, model group (LPS / GalN group), compound ZJU-37 treatment group (5mg / kg ZJU-37+LPS / G...
Embodiment 3
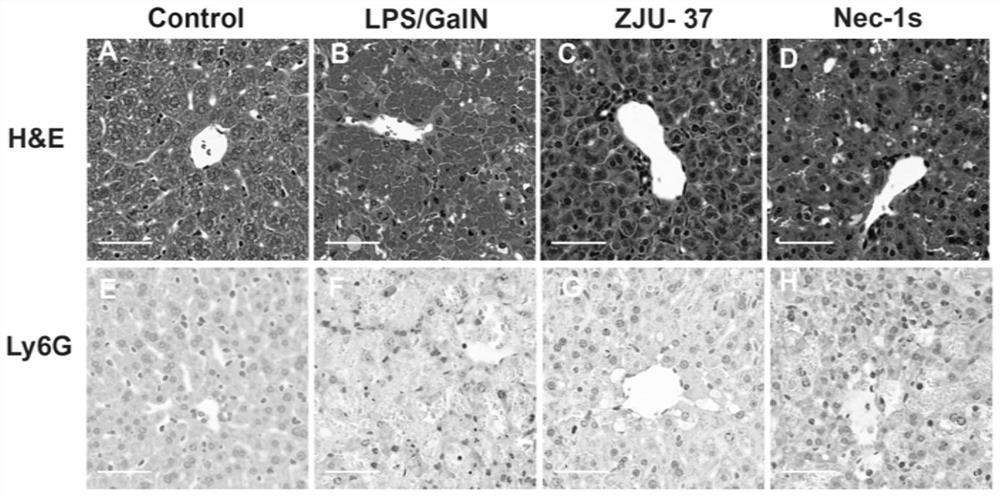
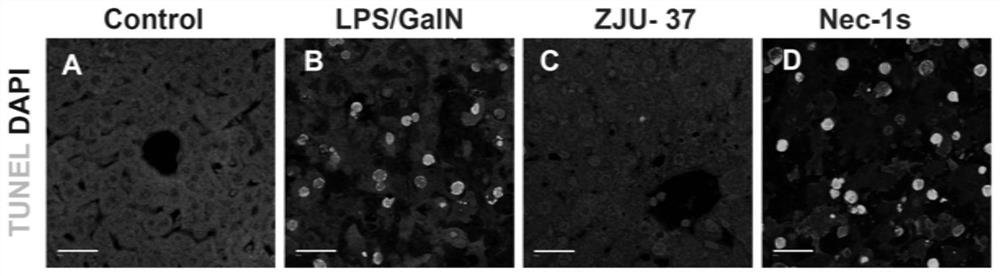
[0035]Example 3 Study of compound ZJU-37 and Nec-1s on liver pathology and neutrophil infiltration in mice with acute liver failure induced by LPS / GalN
[0036]experimental method:
[0037]Six-week-old male C57BL / 6 mice (purchased from Nanjing Institute of Biological Medicine) were randomly divided into normal control group, model group (LPS / GalN group), compound ZJU-37 treatment group (5mg / kg ZJU-37+LPS / GalN group), compound Nec-1s treatment group (5mg / kg Nec-1s+LPS / GalN group). Compound ZJU-37 and Nec-1s were injected intraperitoneally, 30 minutes later, LPS / GalN (40μg / kg LPS+800mg / kg D-GalN) was injected intraperitoneally to build the model; the model group replaced the solvent of compound ZJU-37 with an equal volume of solvent ; The normal control group is not treated. Six hours after LPS / GalN modeling, liver samples were collected for HE and immunohistochemical staining.
[0038]Experimental results:
[0039]The experimental results are asfigure 2 Shown: Figures A and B represent the LPS / ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com