Screening method of wool thiopeptide, cell-free protein synthesis system and wool thiopeptide
A screening method, lanti-peptide technology, which is applied in the field of bioengineering, can solve problems such as limited application, inability to meet production needs, and insufficient activity of lanthipeptide, and achieve simple and fast results
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
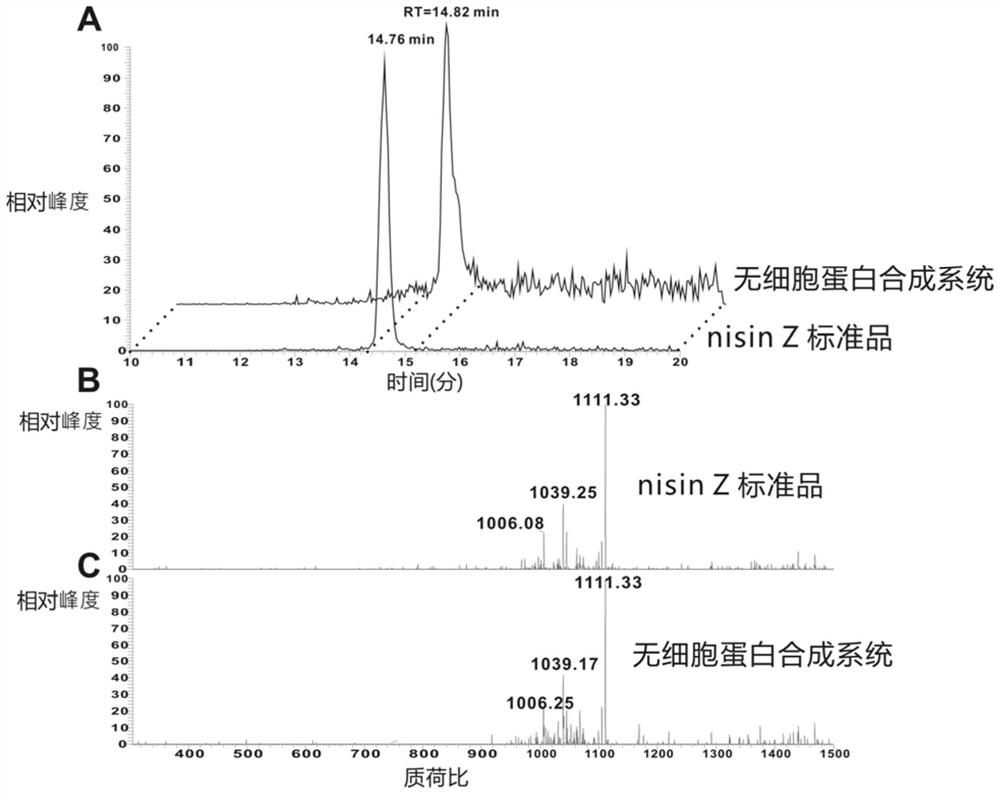
Image
Examples
Embodiment 1
[0044] In this example, construct the plasmids of several key components that regulate the production of nisin, the specific method is as follows:
[0045] (1) Genomic DNA from Lactococcus lactis was obtained using Blood & Cell Culture DNA Mini Kit (QIAGEN, Hileden, Germany) according to the instructions.
[0046] (2) The nisZ sequence nisZ (SEQ ID NO: 1) was artificially synthesized, and then subcloned into pJL1 according to the restriction site nisZ shown in Table 2 to obtain the nisZ expression plasmid.
[0047] Simultaneously, utilize the primer shown in table 1, amplify nisB (SEQ ID NO:2), nisC (SEQ ID NO:3) and nisP (SEQ ID NO:3) and nisP (SEQ ID NO: 4) Gene, then digest the PCR amplified fragment using the restriction sites shown in Table 2, and simultaneously cut the plasmid pET28a with the same enzyme, and select the correct clone after enzyme-linking with T4 ligase , and the positive clones were sequenced and verified, and the expression plasmids of nisB, nisC and n...
Embodiment 2
[0060] Using the nisZ, nisB, nisC and nisP expression plasmids prepared in Example 1 to rebuild the nisin synthesis pathway in vitro, the specific method is as follows:
[0061] 1. Preparation of the main components of the nisin in vitro reaction system (CFPS)
[0062] (1) To obtain NisB and NisC proteins, the method is as follows:
[0063] (a) Escherichia coli BL21(DE3) cells were transformed with plasmids pYL02 and pYL03 respectively, and then single-colony transformants were grown overnight at 37° C. in 50 mL medium supplemented with 50 μg / mL kanamycin.
[0064] (b) Transfer 2L LB medium according to 1% inoculum size until OD600 reaches 0.6-0.8, then cool the culture to 18°C, add IPTG to a final concentration of 0.5mM (NisB) or 0.2mM (NisC) to induce, wherein , for NisC overexpression, add an additional 100 μM ZnCl 2 to ensure its activity.
[0065] (c) After the culture from step (b) was grown in culture for 20 hours, the cells were harvested by centrifugation at 5000 g...
Embodiment 3
[0088] Example 3 Using the nisin core peptide sequence as the conserved sequence, the lanthipeptides conserved with the nisin core peptide sequence were screened out from the database, and the functions of each lanthipeptide were verified with the help of the nisin CFPS system.
[0089] Taking the sequence "SXSLCTPGCXTG" of the nisin core peptide as the conserved sequence, all the sequences predicted to be lanthipeptides conserved with the nisin core peptide region were retrieved from NCBI (as of June 2018), and the "BAGEL4" database ( http: / / bagel4.molgenrug.nl / ) screening to remove known lanthipeptides, and finally screened 18 lanthipeptides of unknown function that were conserved with the core peptide sequence of nisin, and named their core peptide sequences RL1-RL18 respectively.
[0090] Adjust these 18 sequences to conform to the recognition site of NisP (if the N-terminal amino acid of the core peptide sequence is not "IT" or "VT", add "IT" at the N-terminus), and add ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com