Method for removing impurities from nickle solutions by using reusable multi-metal salt as complexing agent
A polymetallic salt, nickel solution technology, applied in the direction of improving process efficiency, can solve the problems of increasing the burden of impurity removal in the subsequent process and failing to achieve deep desilication
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment approach 1
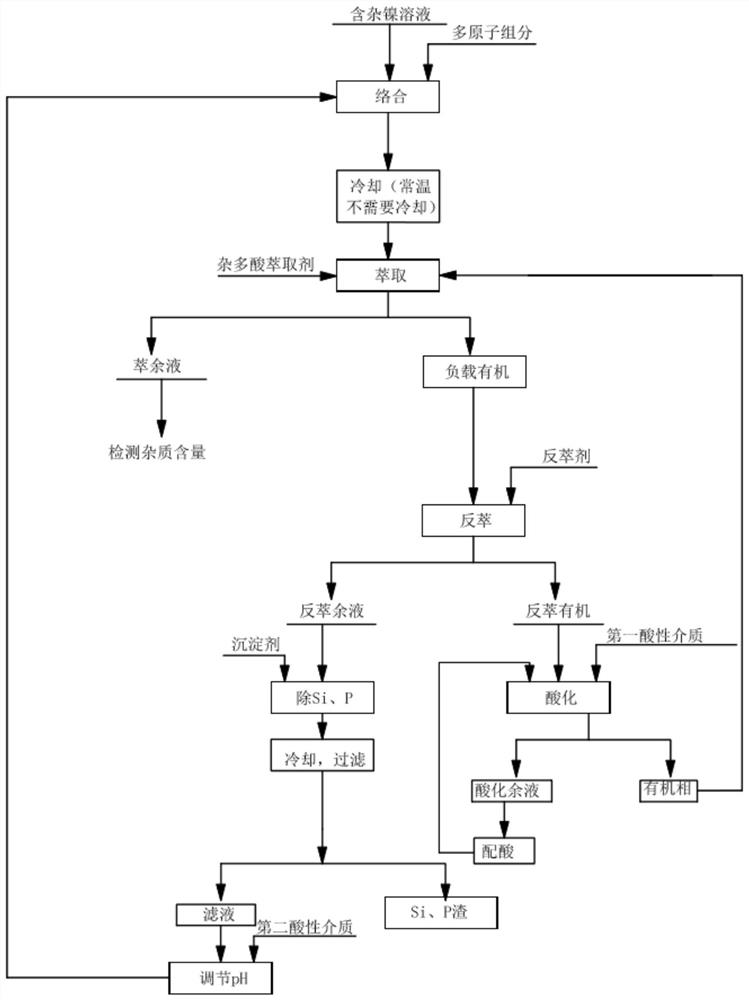
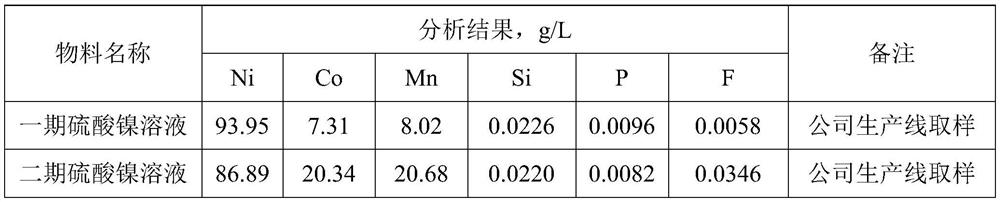
[0138] Add sodium molybdate with 1.5 times the molar content of Si and P heteroelements to the nickel solution, adjust the pH value of the nickel solution to 3.0, stir for 1 hour at room temperature, and then add 10% N235+30% isooctyl alcohol+ 60% kerosene heteropolyacid extractant, the volume ratio of heteropolyacid extractant addition to nickel solution is 1:1, after mixing for 5min, leave to stand for layering, separate and obtain the raffinate (nickel solution) with Si and P impurity content below 1ppm solution) and the organic phase.
Embodiment approach 2
[0140] Add ammonium molybdate with 1.5 times the molar content of Si and P heteroelements to the nickel solution, adjust the pH value of the nickel solution to 4.0, stir for 1 hour under normal temperature conditions, and then add 5% N235+50% sec-octanol+ 45% kerosene heteropolyacid extractant, the heteropolyacid extractant addition and the nickel solution volume ratio are 1:1, after mixing for 10min, leave to stand for layering, separate and obtain the raffinate (nickel solution) with Si and P impurity content below 1ppm solution) and the organic phase.
Embodiment approach 3
[0142] Add potassium molybdate with 1.3 times the molar content of Si and P heteroelements to the nickel solution, adjust the pH value of the nickel solution to 2.0, stir for 2 hours at room temperature, and then add 20% N235+50% n-octanol+ 30% kerosene heteropolyacid extractant, the heteropolyacid extractant addition and the nickel solution volume ratio are 1:1, after mixing for 15min, leave to stand for layering, separate and obtain the raffinate (nickel solution) with Si and P impurity content below 1ppm solution) and the organic phase.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com