Method for detecting drug sex difference adverse reaction signal
A detection method and adverse reaction technology, applied in drug reference, informatics, medical informatics, etc., can solve problems such as difficulty in mining ADR gender differences, lack of female subjects, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
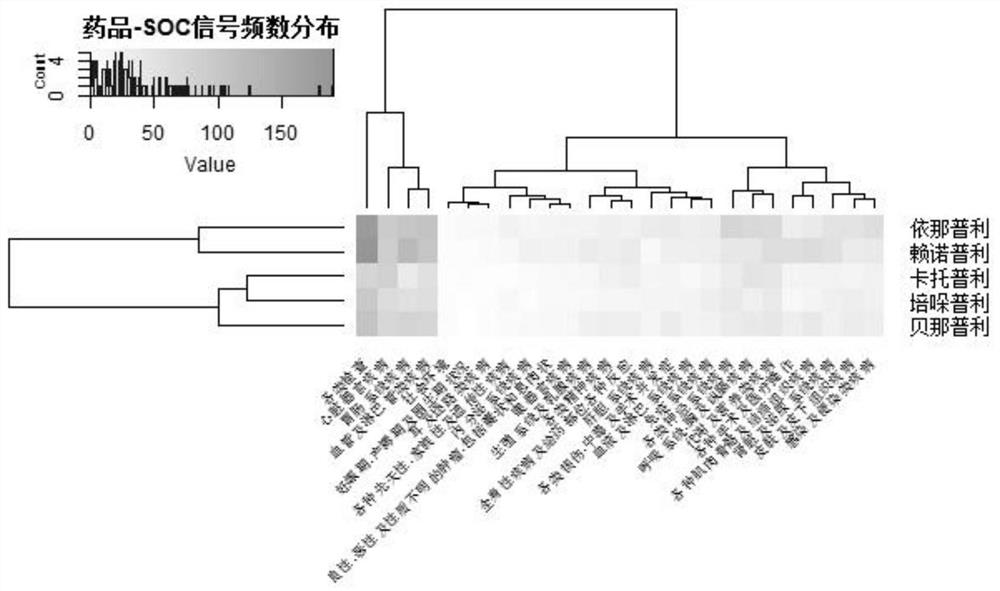
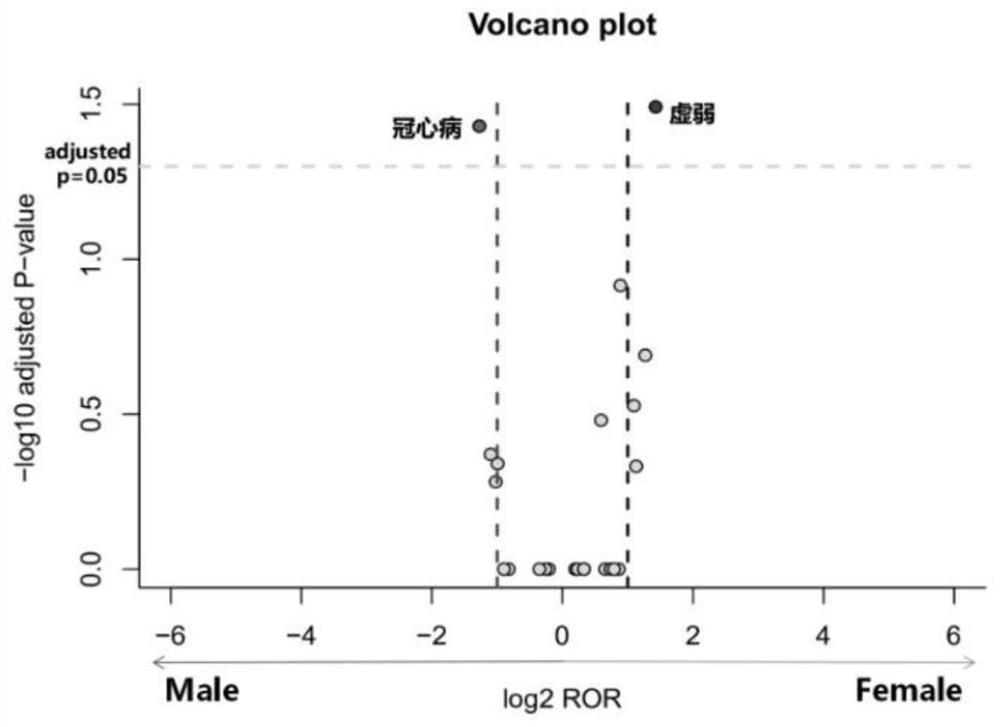
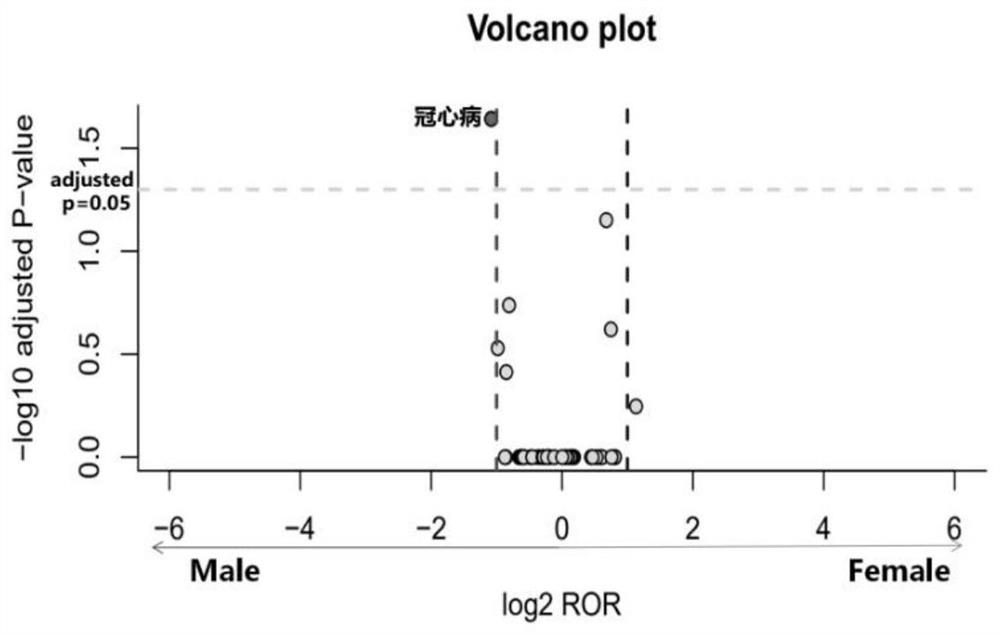
[0035] Data source: The data comes from the Adverse Drug Event (ADE) Reporting System (FDA Adverse Event Reporting System, FAERS) database established by the US Food and Drug Administration (Food and Drug Administration, FDA). The database uses the preferred terms (PreferredTerms, PT) of the "ICH International Medical Dictionary for Regulatory Activities (MedDRA)" (MedDRA) to encode and count ADR, and the raw data are structured. This application included the FAERS database from January 1, 2004 to December 31, 2018 for mining ADR signals involving five ACEI antihypertensive drugs.
[0036] The specific process is as follows:
[0037] 1. Data preprocessing
[0038] Using the OpenVigilFDA analysis tool, the site analysis tool directly obtains the original information in the OpenVigilFDA database through the application program interface API, and performs structured processing, with high extraction efficiency and accuracy. Obtain captopril, benazepril, enalapril, perindopril an...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com