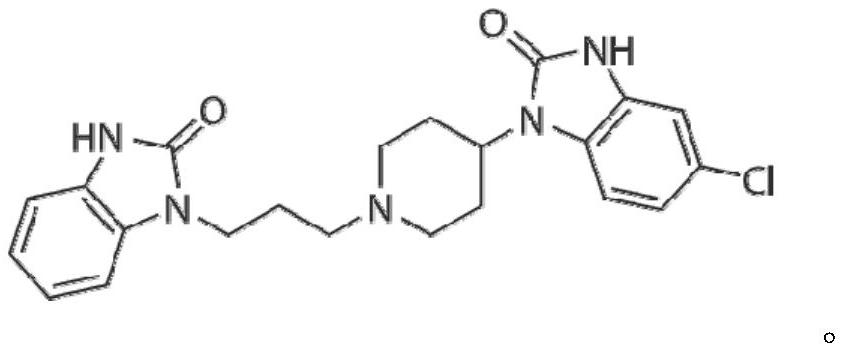
A kind of domperidone tablet and preparation method thereof
A technology of domperidone and diperidone tablets, which is applied in the direction of pharmaceutical formulas, medical preparations with non-active ingredients, medical preparations containing active ingredients, etc., which can solve the problem of unstable content uniformity of active ingredients and unfavorable products to exert their efficacy, etc. problems, to achieve the effect of excellent appearance, low loss and excellent dissolution performance
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
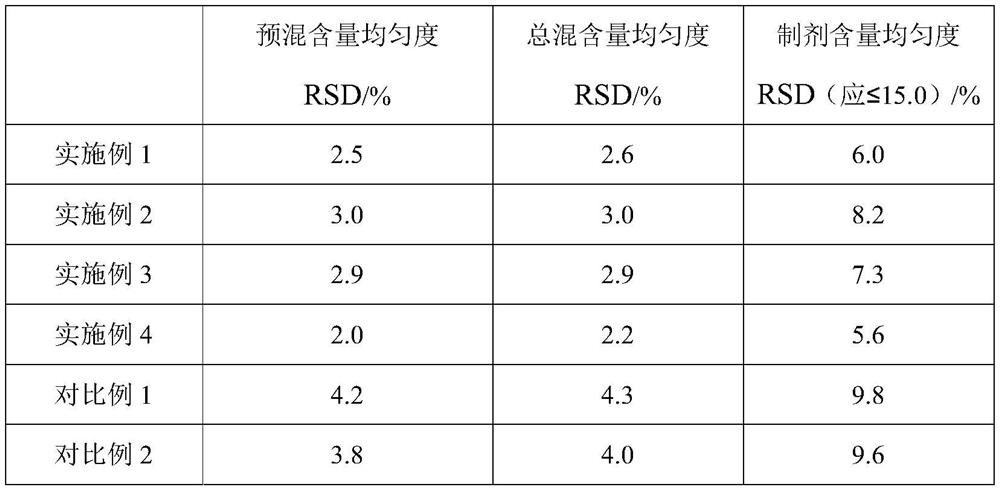
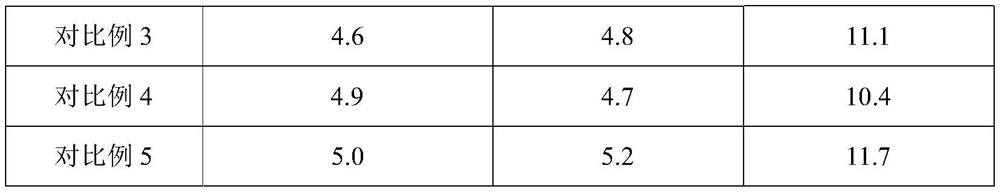
Embodiment 1
[0062] A domperidone tablet, comprising the following components in parts by weight: 1 part of domperidone, 6 parts of lactose, 2 parts of pregelatinized starch, 0.5 part of low-substituted hydroxypropyl cellulose, 0.6 part of povidone K30, dodecane 0.01 part of sodium base sulfate, 0.1 part of silicon dioxide, and 0.08 part of magnesium stearate.
[0063] The preparation technology of above-mentioned domperidone sheet, comprises the steps:
[0064] 1) Premix domperidone with an equal amount of lactose (1 part) for 2 minutes to obtain mixture A;
[0065] 2) Separately take lactose equivalent to that of domperidone, pulverize it in a pulverizer, and pass through a 100-mesh sieve to obtain substance B;
[0066] 3) Pulverize the mixture A in a pulverizer and pass through a 100-mesh sieve for 3 times to obtain the mixture C;
[0067] 4) Pulverize substance B again in a pulverizer, pass through a 100-mesh sieve once, collect the sieved substance B (including the residual mixture ...
Embodiment 2
[0073] A domperidone tablet, comprising the following components in parts by weight: 1 part of domperidone, 10 parts of microcrystalline cellulose, 3.5 parts of starch, 0.8 part of sodium carboxymethyl starch, 0.9 part of polyethylene glycol (molecular weight is 2000) , 0.0045 parts of sodium lauryl sulfate, 0.05 parts of talcum powder, and 0.13 parts of magnesium stearate.
[0074] The preparation technology of above-mentioned domperidone sheet, comprises the steps:
[0075] 1) Premixing domperidone and an equal amount of microcrystalline cellulose for 10 minutes to obtain mixture A;
[0076] 2) Separately take microcrystalline cellulose equivalent to that of domperidone, pulverize it in a pulverizer, and pass through a 60-mesh sieve to obtain substance B;
[0077] 3) Pulverize mixture A in a pulverizer and pass through a 60-mesh sieve to obtain mixture C;
[0078] 4) Pulverize substance B again in a pulverizer, pass through a 60-mesh sieve five times, collect the sieved su...
Embodiment 3
[0084] A domperidone tablet, comprising the following components in parts by weight: 1 part of domperidone, 3 parts of microcrystalline cellulose, 0.5 part of pregelatinized starch, 0.1 part of sodium carboxymethyl starch, 0.3 part of povidone K30, ten parts 0.02 part of sodium dialkylsulfonate, 0.15 part of sodium stearyl fumarate, and 0.03 part of magnesium stearate.
[0085] The preparation technology of above-mentioned domperidone sheet, comprises the steps:
[0086] 1) Premixing domperidone with an equal amount of microcrystalline cellulose for 5 minutes to obtain mixture A;
[0087] 2) Separately take microcrystalline cellulose equivalent to that of domperidone, pulverize it in a pulverizer, and pass through an 80-mesh sieve to obtain substance B;
[0088] 3) Pulverize mixture A in a pulverizer and pass through an 80-mesh sieve to obtain mixture C;
[0089] 4) Pulverize the substance B again in the pulverizer, pass through the 80-mesh sieve three times, collect the sie...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com