Epitope peptide of tropomyosin antigen, and application of epitope peptide
A tropomyosin and epitope peptide technology, which is applied to the epitope peptide of tropomyosin antigen and its application field, can solve the problem that mollusk allergens are less than crustacean allergens, etc., achieve good application prospects, reduce pathogenic Sensitivity effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
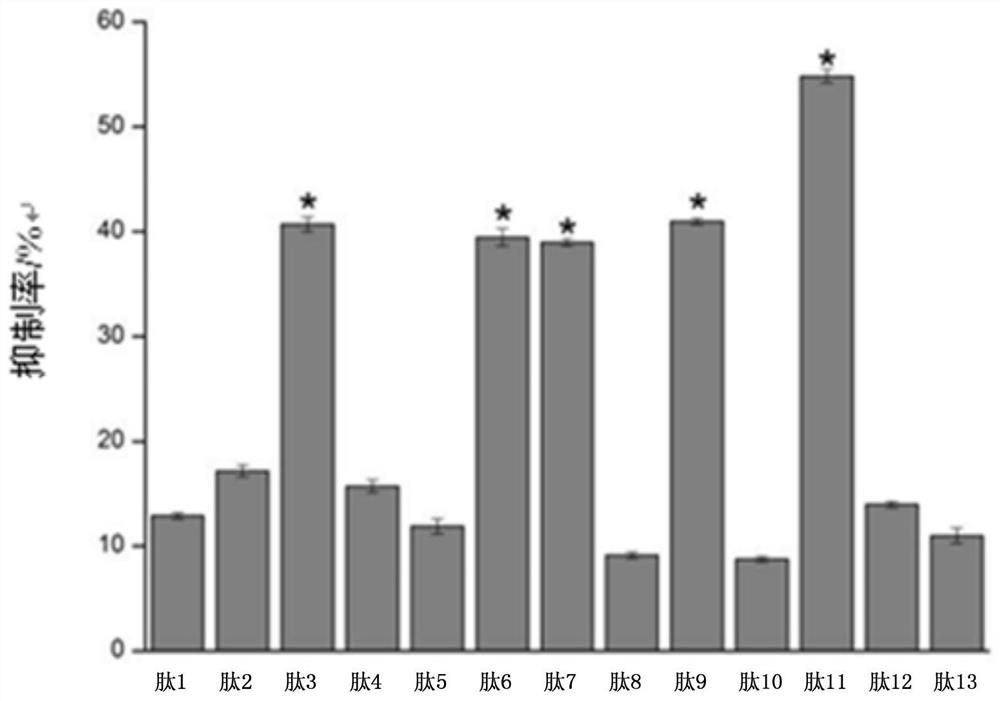
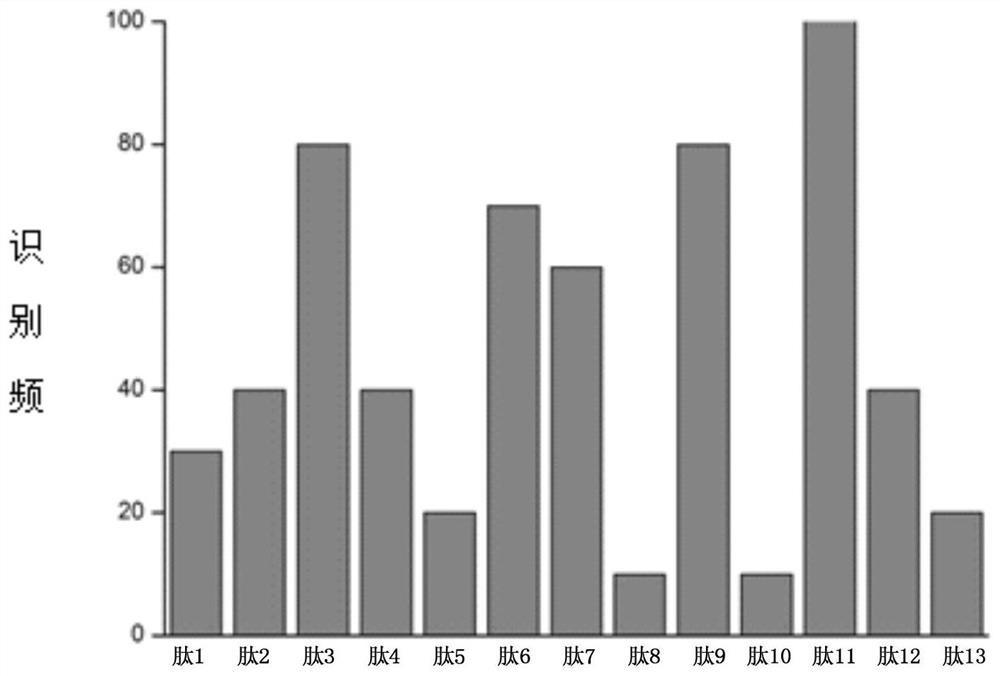
[0032] Example 1 Detection of whether there is an antibody to the tropomyosin antigen of the present invention in the sample
[0033] 1. Materials and Methods
[0034] Serum samples from 10 subjects with oyster allergy were obtained from Plasma Lab International, USA. All 10 subjects had a clinical history of at least two or more symptoms within 1 h of ingesting oysters, including pruritus, allergic rhinitis, gastrointestinal disorders, and diarrhea. Venous blood from 10 patients with oyster allergy was collected and pooled to prepare a serum pool. Serum samples (from Plasma Laboratories International, USA) from 5 normal volunteers without any history of shellfish allergy were used as negative controls. Each serum sample was stored in aliquots at -80°C until use.
[0035] Experimental serum pool: Serum samples from 10 patients with oyster allergy were mixed in equal volumes.
[0036] Control serum pool: 5 serum samples without any history of shellfish allergy were mixed in...
Embodiment 2
[0058] Construction of the modified tropomyosin antigen of embodiment 2
[0059] All 5 IgE-binding epitopes were deleted to construct 5 peptide-deleted modified tropomyosin antigens, which were named as CrD224. The mutant only contained 224 amino acid residues, specifically, it was identified by SEQ ID No.14 Amino acid sequence shown:
[0060] SEQ ID No. 14:
[0061] MDSIKKKMIAMKMEKENAQDRAEQLEQQLRDTEEQKAKIEEDLHSNLENEFDTVNEKYQECQQSLNRRIQLLEEDATEKLEEASKAADESERNRKVLENLEKQLTEAKLIAEEADKKYDEAARKLAITEVDLERAEARLEAAEAKVYELEEQLSVVANNIKTLQIRDLTQRLKDAENRATEAERTVSKELAGERQKEVDR
[0062] The specific experimental process for constructing modified tropomyosin antigens with five peptide deletion mutations is as follows:
[0063] The entire protein sequence of Cra g 1 contains 284 amino acids, and the above-mentioned 5 specific IgE binding sequences (peptides 11, 9, 3, 6, 7) are deleted in the entire protein sequence, and the specific amino acid sequence is located in Cra g 1 44-49 , Crag 1 ...
Embodiment 3
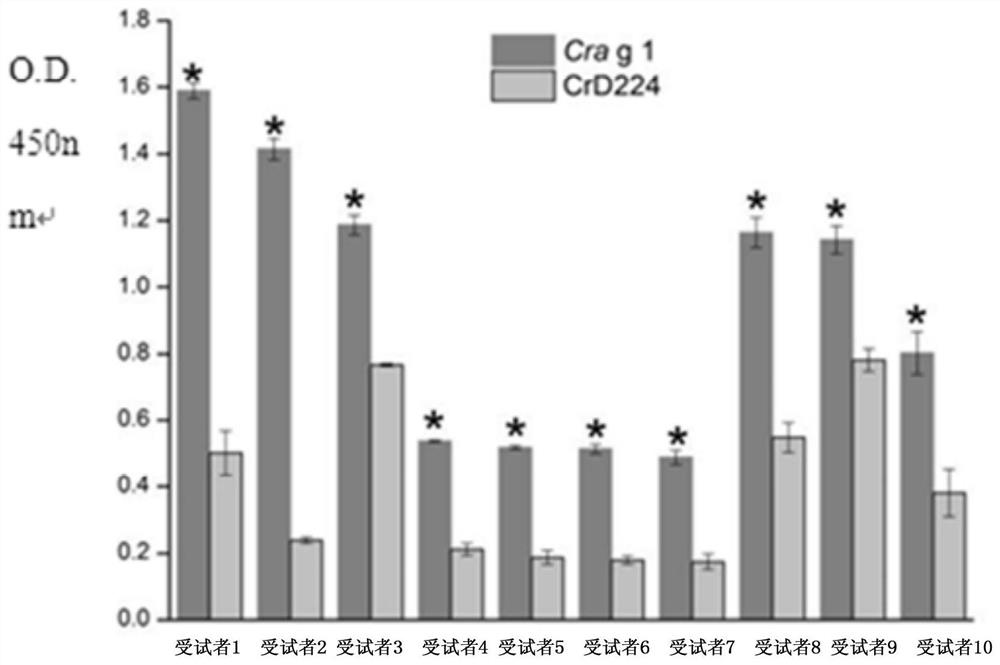
[0064] Comparison of the reactivity of antigen Cra g 1 and its mutant CrD224 (i.e. modified tropomyosin antigen) with serum IgE in embodiment 3
[0065] 1. Experimental materials: the blood of 10 oyster allergic subjects, and the serum sources of 5 normal volunteers without any history of shellfish allergy are the same as in Example 1.
[0066] Experimental serum pool: equal volumes of serum from 10 subjects with oyster allergy were mixed and prepared.
[0067] Control serum pool: 5 serums without any history of shellfish allergy were prepared by mixing equal volumes.
[0068] Mouse sera: 10 mouse sera were obtained from Plasma Lab International.
[0069] 2. Synthesis of Epitope Peptides
[0070] The sequence and synthesis of the five peptides of SEQ ID No.1-5 and each reference peptide are the same as in Example 1.
[0071] 3. Comparison of the reactivity of antigen Cra g 1 and its mutant CrD224 with serum IgE
[0072] ① Antigen coating: First, dilute the antigen (Crag 1 ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com