Preparation method of 3',5'-dichloro-2,2,2-trifluoroacetophenone
A technology of trifluoroacetophenone and chlorinating reagent is applied in the field of preparation of 3′,5′-dichloro-2,2,2-trifluoroacetophenone, and can solve the problem of high industrial production cost and high raw material cost , poor economic effect and other problems, to achieve the effects of low equipment maintenance components, lower production costs, and improved economic effects
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
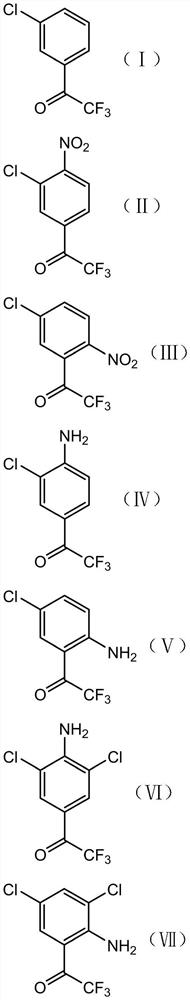
[0051] A preparation method of 3',5'-dichloro-2,2,2-trifluoroacetophenone, comprising the steps of:
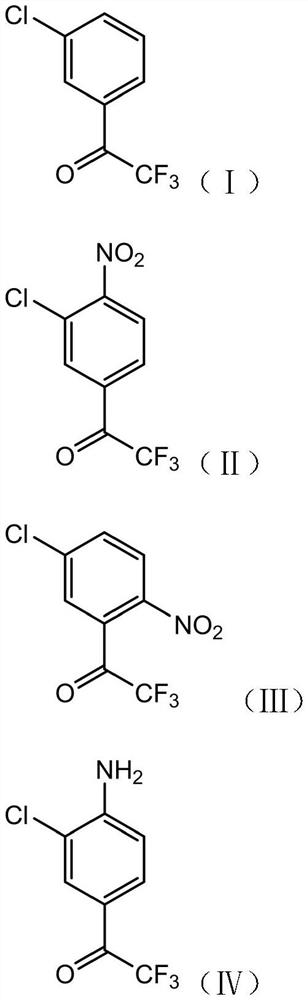
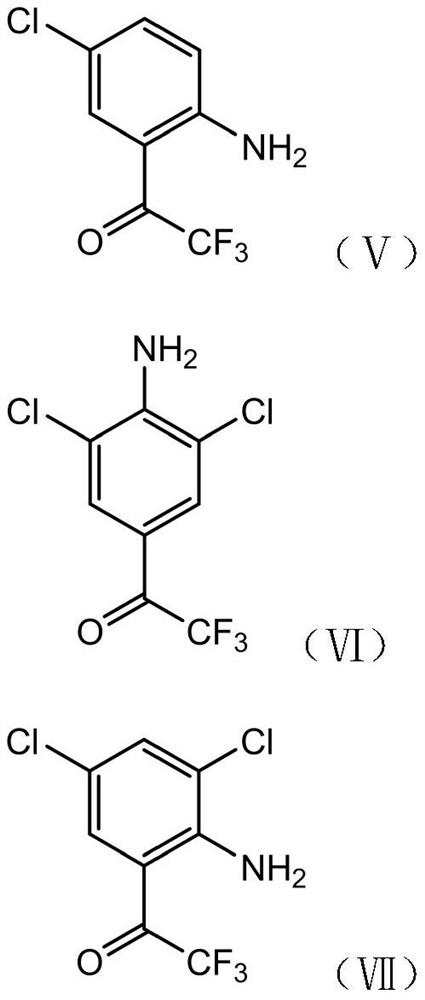
[0052] S1. Nitrating reaction of compound I with a nitrating reagent under the catalysis of acid I to obtain a composition of compound II and compound III;
[0053] S2, subjecting the composition of compound II and compound III obtained in step S1 to a nitro reduction reaction to obtain a composition of compound IV and compound V;
[0054] S3. Chlorinating the composition of compound IV and compound V obtained in step S2 with a chlorination reagent to obtain a composition of compound VI and compound VII;
[0055] S4, performing diazotization elimination reaction on the composition of compound VI and compound VII obtained in step S3 to obtain 3′,5′-dichloro-2,2,2-trifluoroacetophenone;
[0056]
[0057]
[0058] Step S1 specifically includes the following sub-steps:
[0059] S1-1. Take 104.5 g (0.5 mol) of compound I, stir and mix with 200 g of concentrated sulfuric aci...
Embodiment 2
[0073] A preparation method of 3′,5′-dichloro-2,2,2-trifluoroacetophenone, the difference from Example 1 is that in step S1-1, acid I is selected from fuming sulfuric acid (200g, 04mol).
Embodiment 3~4
[0075] A preparation method of 3′,5′-dichloro-2,2,2-trifluoroacetophenone, the difference from Example 1 is that in step S1-1, acid I uses 80% sulfuric acid, heat preservation reaction The time is 2h and 1h respectively.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com