Method for extracting paracrine factor from adipose-derived stem cells
A technology of adipose stem cells and adipocytes, which is applied in the preparation methods of cytokines/lymphokines/interferons, peptides, animal cells, etc., and can solve the problems of unscaled separation and purification process, small culture scale, and uneven quality levels , to achieve the effect of reducing cell differentiation problems, stable karyotype, and reducing research errors
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
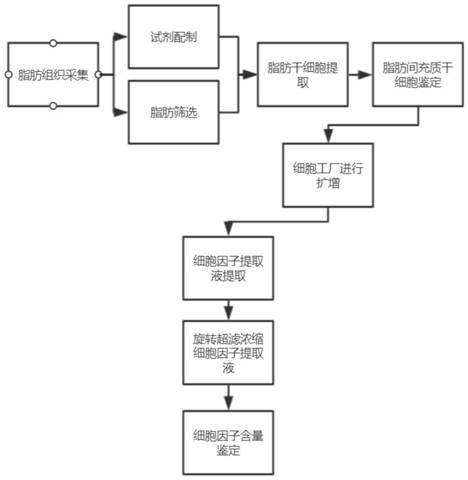
[0075] Example 1 Extraction of paracrine factors from adipose stem cells
[0076] Fat cell digestion solution is composed of DMEM / F12 medium (DMEM medium and F12 medium are mixed at a volume ratio of 1:1) and type I collagenase, and the concentration of type I collagenase is 0.1% to 0.3% % (percentage by weight).
[0077] The paracrine factor extract is composed of PBS buffer, L-glutamine, D-glucose and L-ascorbic acid, wherein the concentrations of L-glutamine, D-glucose and L-ascorbic acid are respectively: 2mmol / L, 15μmol / L, 50μmol / L.
[0078] Proceed as follows:
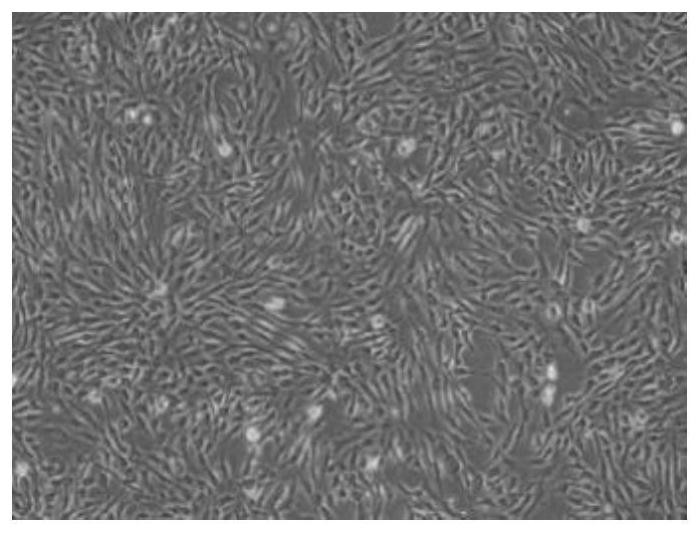
[0079] (1) Primary Extraction of Adipose Stem Cells
[0080] (1) Take the fat sample collected and centrifuge (800g, 8min);
[0081] The fat specimens are subcutaneous fat collected from the thighs and abdomen below the umbilicus of the subjects (not limited to sex, age under 35 years old). The fat tissue content in these parts is rich, and the fat particles are fine, uniform and pure; , the subject is plac...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com