Chiral thermal activation delayed fluorescence material and preparation method thereof
A technology of thermally activated delay and fluorescent materials, which is applied in the direction of luminescent materials, organic chemical methods, chemical instruments and methods, etc., and can solve the problems of low yield, difficulty in large-scale mass production, and few types of materials.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0070] The reaction formula is as follows:
[0071]
[0072] The reaction is as follows:
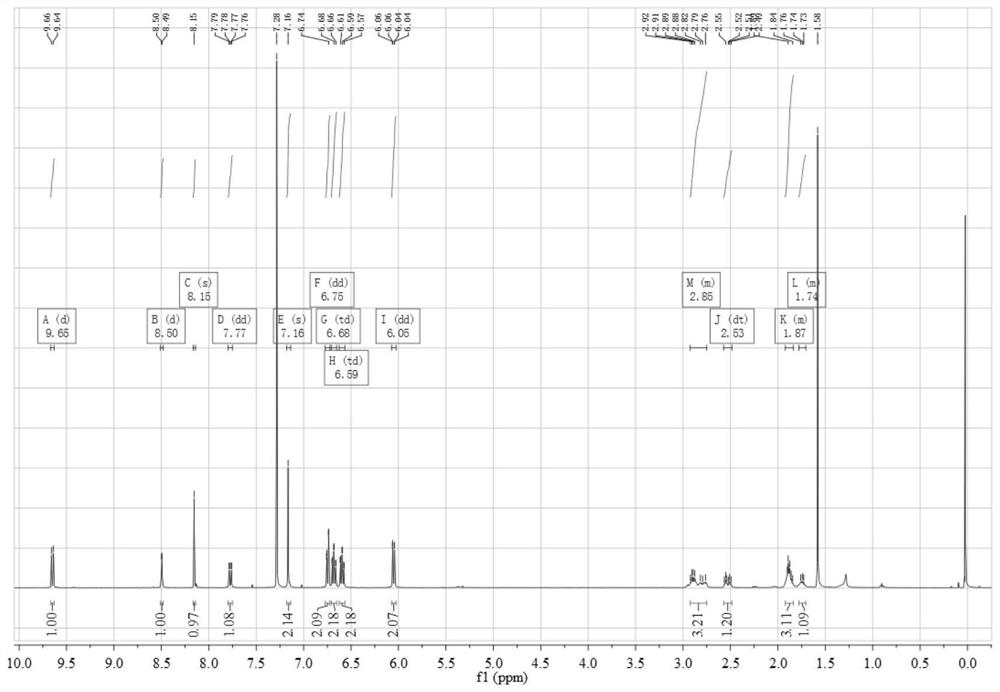
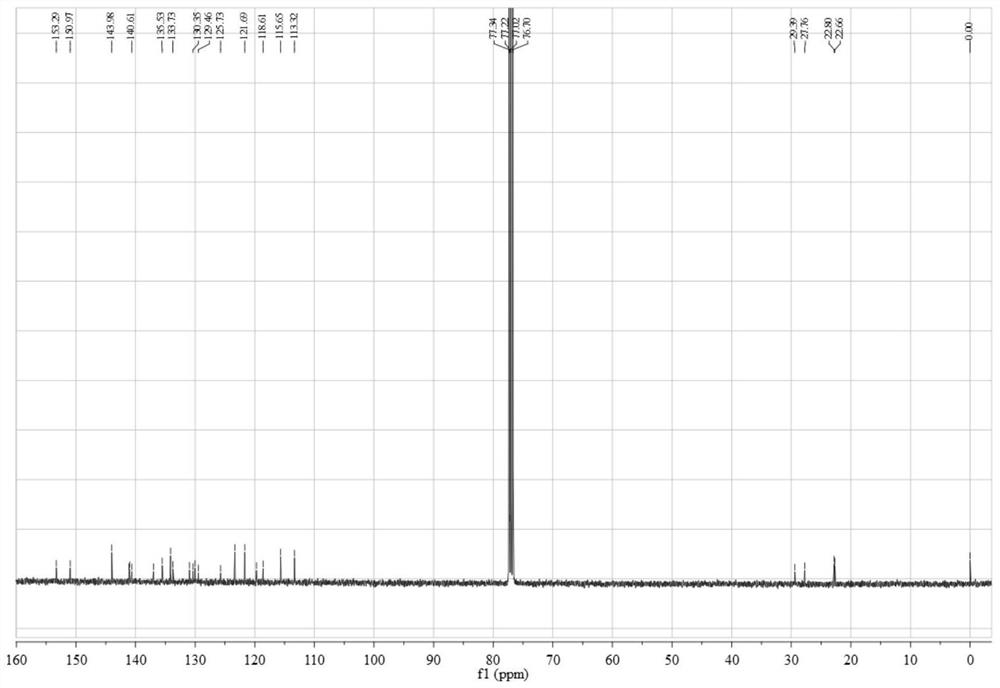
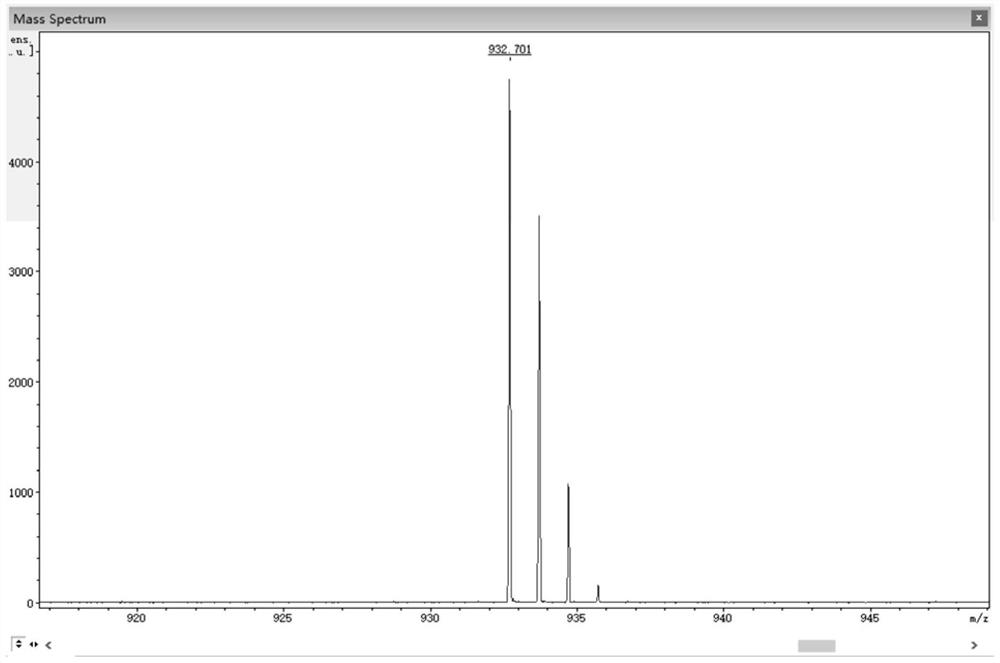
[0073]Add 0.45 g (3.12 mmol) of 4,5-difluorobenzene-1,2-diamine and 1.12 g (3.06 mmol) of 3,6-dibromo-9,10-phenanthrenequinone into a 150 mL three-necked flask, and then add 100 mL of absolute ethanol as a solvent, stirred under the protection of nitrogen, reacted at 80 °C for 2 hours, then filtered the reaction solution, and recrystallized the filter cake with absolute ethanol to obtain a light yellow solid 3,6-dibromo-11,12-di Fluorodibenzo[a,c]phenazine in 95% yield.
[0074] 0.50 g (1.05 mmol) 3,6-dibromo-11,12-difluorodibenzo[a,c]phenazine, 0.40 g (2.18 mmol) 10H-phenoxazine, 0.41 g (4.27 mmol) sodium tert-butoxide, 0.016 g (0.055 mmol) tri-tert-butylphosphine tetrafluoroborate, 0.05 g (0.055 mmol) tris(dibenzylideneacetone) dipalladium (0), then add 50 mL toluene As a solvent, the reaction was heated at 100 °C under the protection of nitrogen; after the reaction was completed...
Embodiment 2
[0082] The reaction formula is as follows:
[0083]
[0084] The reaction is as follows:
[0085] 0.45 g (3.12 mmol) 4,5-difluorobenzene-1,2-diamine and 1.12 g (3.04 mmol) 1,2-bis(4-bromophenyl)ethane-1 were added to a 150 mL three-neck flask, 2-diketone, then add 100 mL of absolute ethanol as a solvent, stir under the protection of nitrogen, react at 80 °C for 2 hours, then filter the reaction solution, and recrystallize the filter cake with absolute ethanol to obtain a light yellow solid 2,3- Bis(4-bromophenyl)-6,7-difluoroquinoxaline, yield 95%.
[0086] 1.00 g (2.10 mmol) 2,3-bis(4-bromophenyl)-6,7-difluoroquinoxaline, 0.80 g (4.36 mmol) 10H-phenoxazine, 1.00 g (10.4 mmol) sodium tert-butoxide, 0.032 g (0.11 mmol) tri-tert-butylphosphine tetrafluoroborate, 0.10 g (0.11 mmol) tris(dibenzylideneacetone) dipalladium (0), and then add 50 mL toluene for Solvent, heat reaction at 100 ℃ under the protection of nitrogen; after the reaction is completed, extract with 100 mL o...
PUM
| Property | Measurement | Unit |
|---|---|---|
| external quantum efficiency | aaaaa | aaaaa |
| dissymmetry factor | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com