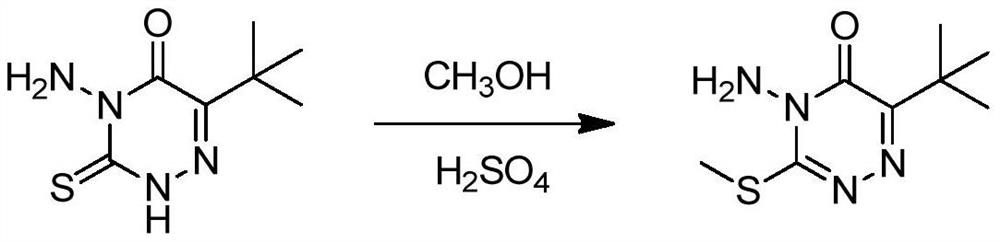
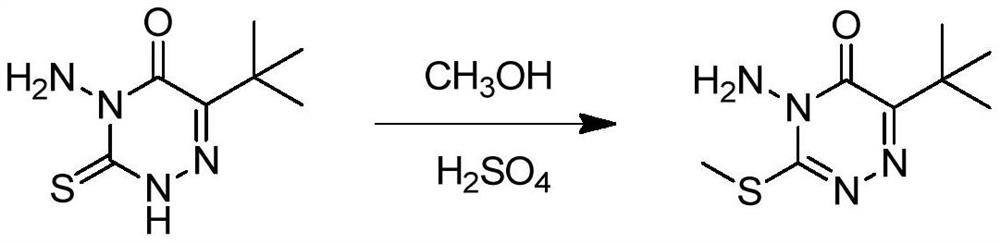
Metribuzin synthesis method
A synthesis method and technology of mezotrione are applied in the field of efficient mezotrione synthesis, and can solve the problems of high cost of process raw materials, low content, long reaction time and the like
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0063] Add 200 grams of 6-tert-butyl-4-amino-3-mercapto-1,2,4-triazin-5(4H)-one to 400 grams of 98% sulfuric acid, heat to completely dissolve and clarify, then cool down to 40°C , add 39 grams of methanol dropwise, after the dropwise addition, heat up to 70-75°C and keep the temperature for 7 hours, then cool down to 20-30°C, add 200g of water dropwise, at this time a large amount of material precipitates out, which is metrizone sulfate. The sulfate was suspended in 300 grams of toluene, then added dropwise with 30% sodium hydroxide solution to neutralize to a pH of about 9, allowed to stand for stratification, and the upper organic phase was cooled to 0°C to crystallize, filtered, and dried to obtain 190 grams Mecitrione, the content is 98.6%.
Embodiment 2
[0065] In 400 grams of 98% sulfuric acid, 39 grams of methanol was added dropwise, and after cooling to 40°C, 200 grams of 6-tert-butyl-4-amino-3-mercapto-1,2,4-triazine-5 ( After adding 4H)-ketone, raise the temperature to 70-75°C and keep it warm for 7 hours, cool down to 20-30°C, add 200 grams of water dropwise, at this time a large amount of material precipitates out, which is metrizone sulfate, and this sulfate Suspended in 300 grams of toluene, then added dropwise 30% sodium hydroxide solution to neutralize to a pH of about 9, allowed to stand for stratification, cooled the upper organic phase to 0°C to crystallize, filtered, and dried to obtain 188 grams of azimidone , content 98.5%.
Embodiment 3
[0067] Add 200 grams of 6-tert-butyl-4-amino-3-mercapto-1,2,4-triazin-5(4H)-one to 400 grams of 98% sulfuric acid, heat to completely dissolve and clarify, then cool down to 40°C , add 39 grams of methanol dropwise, after the dropwise addition, heat up to 70-75°C and keep the temperature for 7 hours, then cool down to 20-30°C, add 200g of water dropwise, at this time a large amount of material precipitates out, which is metrizone sulfate. The sulfate was suspended in 300 grams of xylene, then added dropwise with 30% sodium hydroxide solution to neutralize to a pH of about 9, allowed to stand for stratification, and the upper organic phase was cooled to 0°C to crystallize, filtered, and dried to obtain 185 Chrysintrione, content 98.2%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com