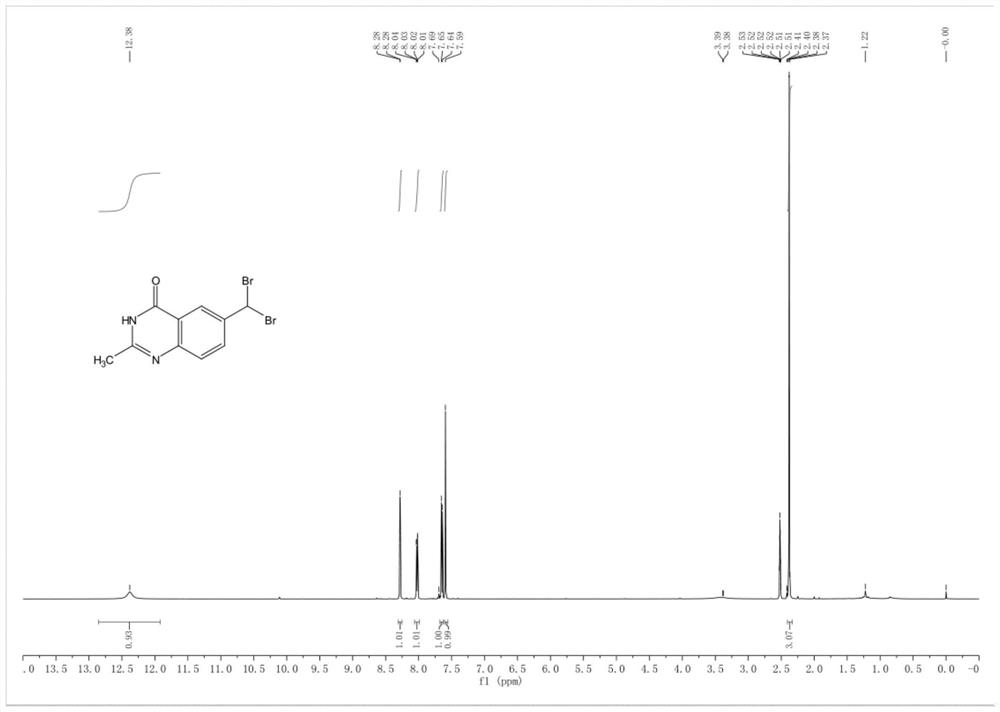
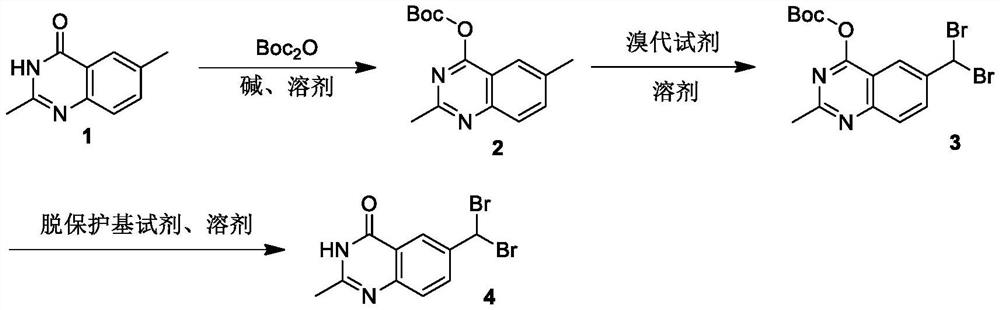
Synthesis method of 6-(dibromomethyl)-2-methylquinazoline-4(3H)-ketone
A technology of dimethylquinazoline and methylquinazoline is applied in the field of synthesis technology of pharmaceutical intermediates and achieves the effects of high yield, convenient operation and improved solubility
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0035] The first step: the synthesis of tert-butyl (2,6-dimethylquinazolin-4-yl) formate (2)
[0036] Take a 500mL three-necked flask, add 2,6-dimethylquinazolin-4(3H)-one (1) (10g, 57.40mmol), add anhydrous THF (300mL) and lower the temperature to within 10°C under nitrogen protection, then divide Sodium hydride (11.48 g, 287.00 mmol) was added in portions. After the addition, remove the ice bath, slowly rise to room temperature, add Boc 2 O (15.03g, 68.88mmol), then the temperature was raised to 50-60°C and the reaction was stirred for 3 hours, and the reaction was complete as detected by TLC. The system was cooled down to 0-10°C and quenched with water, then extracted with ethyl acetate, the organic phases were combined, dried over anhydrous sodium sulfate, filtered, and the filtrate was concentrated under reduced pressure to remove the solvent. Add silica gel to mix the sample, and purify by column chromatography (eluent: petroleum ether: ethyl acetate=20:1) to obtain te...
Embodiment 2
[0042] The first step: the synthesis of tert-butyl (2,6-dimethylquinazolin-4-yl) formate (2)
[0043] Take a 500mL three-necked flask, add 2,6-dimethylquinazolin-4(3H)-one (1) (10g, 57.40mmol), add anhydrous THF (300mL) and lower the temperature to below -10°C under nitrogen protection. Add LDA (2mol / L, 34mL, 68.88mmol) and keep the reaction for about 0.5 hours. After the addition, it was slowly raised to room temperature, and Boc was added 2O (15.03g, 68.88mmol), then the temperature was raised to 50-60°C and the reaction was stirred for 3 hours, and the reaction was complete as detected by TLC. Cool the system down to 0-10°C and add water to quench it, add ethyl acetate to extract, combine the organic phases, dry over anhydrous sodium sulfate, filter, concentrate the filtrate under reduced pressure to remove the solvent, add silica gel to mix the sample, and purify by column chromatography (eluent: Petroleum ether: ethyl acetate = 20:1) to obtain tert-butyl (2,6-dimethylqu...
Embodiment 3
[0049] The first step: the synthesis of tert-butyl (2,6-dimethylquinazolin-4-yl) formate (2)
[0050] In a 250mL reaction flask, add DMF (60mL), add 2,6-dimethylquinazolin-4(3H)-one (1) (2.00g, 11.48mmol) under stirring, nitrogen protection, and then add hydrogen in sequence Potassium oxide (3.21g, 57.40mmol), Boc 2 O (3.01g, 13.78mmol), then heated up to 50-60°C and stirred for 2-3h, TLC reaction was complete, then cooled to 0-10°C, filtered to remove inorganic salts, added water and ethyl acetate to extract layers, combined The organic phase was dried and filtered using anhydrous sodium sulfate, the filtrate was concentrated under reduced pressure to remove the solvent, the sample was mixed with silica gel, and purified by column chromatography (eluent: petroleum ether: ethyl acetate = 20:1) to obtain compound 2 (yield 2.52g , yield 80.0%).
[0051] The second step: the synthesis of tert-butyl (6-(dibromomethyl)-2-methylquinazolin-4-yl) formate (3) in the 100mL reaction fl...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com